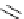Chemistry Reference
In-Depth Information
NH
2
O
O
R
H
2
N
H
2
N
OTBDMS
O
R
HN
R
NH
2
TBDMSO
TBDMS=
t
Bu(Me)
2
Si
OTBDMS
H
+
N
NN
H
O
O
R
R
10
Scheme 4.7
Example of the preparation of bicyclic guanidines with spiro rings
2-hydroxyethyl-substituted guanidines to 2-amino-1,3-imidazolidine systems, in which
chlorination of the primary alcohol function followed by intramolecular substitution
reaction occurs, for other types of disubstitutedmonocyclic and bicyclic guanidines [27c]
(Scheme 4.8c).
4.3 Guanidines as Synthetic Tools
There are many reports on the synthetic uses of TMG (1) and its analogues such as Barton
s
base (2). In this section, their synthetic roles in organic synthesis will be discussed
according to their tentative classification into three categories [addition (catalytic reaction),
substitution (stoichiometric reaction) and others] from the view points of a landmark for
guanidine mediated asymmetric synthesis.
4.3.1 Addition
4.3.1.1 Aldol-Type Reaction
Carbonyl Substrate
Alkyl phosphonates are prepared smoothly by TMG (1) catalysed aldol-type addition of
dialkyl phosphites to ketones and imines under mild conditions [28] (Scheme 4.9). Dialkyl
phosphites can also serve as good nucleophiles for Michael addition (phospha-Michael
Reaction).
TBD (3a) and its 7-methyl derivative (3b) were proven to be powerful catalysts, in many
cases superior to TMG (1), in the addition of dialkyl phosphites to a variety of carbonyl
compounds [29]. The polymer-supported (PS) TBD was also proven to be an efficient
catalyst.