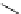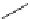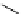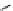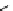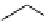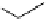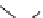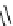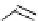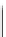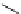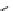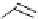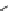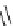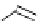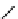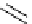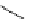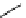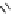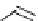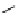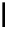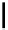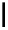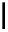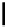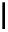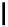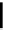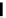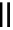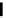Chemistry Reference
In-Depth Information
Ph
O
Ph
O
H
H
N
N
N
N
Me
H
H
NH
O
O
H
N
NH
2
Me
N
N
N
H
H
8
NH
H
2
N
(a)
S
-Methylisothioureas
(b) Protedted guanidines
SMe
NHR
NR
NTf
RHN
RHN
R
= Boc
R
= Cbz
R
= 2-X-C
6
H
4
CH
2
OCO (X = Cl, Br)
R
= Boc
R
= Cbz
R
= allyloxycarbonyl
(c) Pyrazole-1-caboxamidines
(d) Benztriazole-1-caboxamidines
R
1
R
1
N
N
N
R
2
N
R
2
N
NR
5
R
3
R
4
N
NR
5
R
3
R
4
N
R
1
= Cl, R
2
= R
3
= H, R
4
= R
5
= Boc
R
1
= R
3
= H, R
2
= NO
2
, R
4
= R
5
= Boc
R
1
= R
2
= R
3
= H, R
4
= Boc, R
5
= Ts
R
1
= R
2
= Me, R
3
= R
4
= H, R
5
= NO
2
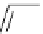
Scheme 4.4 Preparation of monosubstituted acyclic guanidine 8 and selected guanidinylating
reagents of amines
triflyl guanidine attached to the resin via a carbamate linker, allows for rapid synthesis of
guanidines from a variety of amines [19].
N,N
0
,N
00
-Tri(Boc)-guanidine and N,N
0
,N
00
-tri(Cbz)-guanidine allow for the facile con-
version of alcohols to substituted guanidines under Mitsunobu condition [20].
4.2.3 Bicyclic Guanidines
Bicyclic guanidines [21] are basically prepared according to the method through the
thiourea intermediate shown in Scheme 4.2 (Scheme 4.5).