Environmental Engineering Reference
In-Depth Information
LUMO levels of active layer materials are very important issue for molecular
design. For organic semiconductors, HOMO and LUMO can be tuned by modi-
fying their backbones or side groups. Poly(p-phenylene) (PPP) and its derivatives,
like polyfluorene (PF), are not electron-rich materials, and hence HOMO levels of
this kind of polymers are quite low (typically below -5.3 eV); polymers or
oligomers built by electron-rich conjugated components, like thiophene or pyrrole,
exhibit much strong electron donating property and hence high HOMO levels. Side
groups (substituents on the backbones) have great influence on molecular energy
levels of organic semiconductors. The comparison of molecular energy levels of
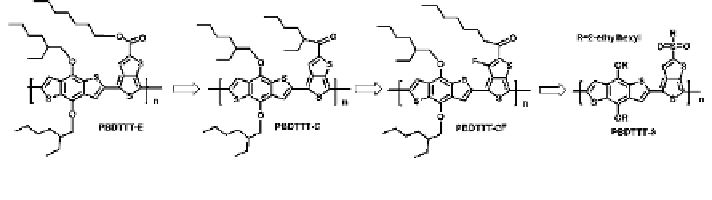
PBDTTT-E, PBDTTT-C, PBDTTT-CF, and PBDTTT-S provides a good example
for the effect of side groups. As shown in Scheme
2.4
, these four polymers have
identical conjugated backbones and different side groups. The basic properties of
these four polymers are shown in Table
2.1
. From PBDTTT-E to PBDTTT-S, the
electron withdrawing effect of side groups were increased stepwise (electron
withdrawing effect: ester \ carbonyl \ carbonyl ? F \ sulfonyl), and therefore,
the HOMO and LUMO levels were gradually lowered.
2.1.3 Mobility Improvement
To improve mobility of an organic semiconductor is a more complicated task, than
to modulate its band gap and molecular energy level. In order to get good mobility,
several issues should be considered during molecular structure design. Inorganic
semiconductors have well-defined crystalline structure and the charges (hole or
electron) can be transported easily through the conduction band; for organic
semiconductor, the charges are localized due to their low dielectric constants.
Therefore, organic semiconductors have much lower mobilities than inorganic
semiconductors. Since, the molecules of an organic semiconductor are stacked
together by weak forces, like van der Waals force, and the charges are transported
through a hopping mode, compact stacking is necessary to facilitate the inter-
molecular charge transport. For example, regioregular poly(3-alkylthiophene)
shows much better hole mobility than its regioregular analog [
3
].
Band gap, molecular energy level, mobility, solubility, and the other issues of
conjugated polymers and small molecular materials are quite susceptible to their
Scheme 2.4
Molecular structures of four PBDTTTs
Search WWH ::

Custom Search