Environmental Engineering Reference
In-Depth Information
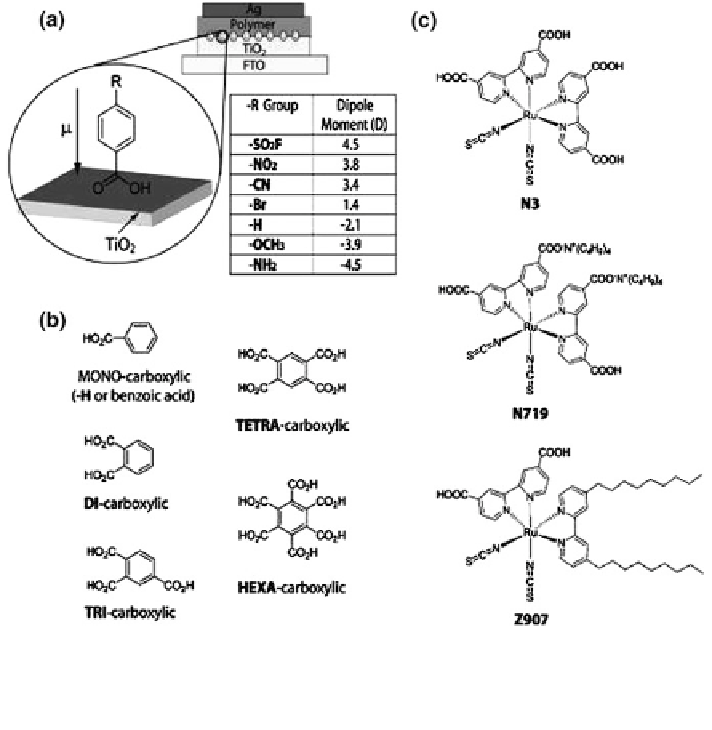
Fig. 9.15 a Schematic of the interface modification of the bilayer TiO2/polymer solar cell. The
table lists the calculated dipole of the benzoic acid with different R group. b molecular structures
of benzene carboxylic molecules. c molecular structure of Ruthenium dyes. Reproduced with
permission from Ref. [
105
]
Interface modification was also reported to affect the crystallinity of the
interfacial P3HT layer in a bilayer ZnO/P3HT device. The crystallinity of P3HT
was decreased when it was casted on ZnO surface as identified by the blue shift
(50 nm) of its absorption peaks. Upon modification by alkanethiol, the crystallinity
of P3HT was recovered and enhanced J
sc
was observed since the more ordered
P3HT has broader absorption spectrum and higher hole mobility [
108
]. Similar
effects were also reported for the Ruthenium dye [
22
].
Ruthenium dyes are most commonly used interface modifiers. However, the
surface of inorganic acceptors cannot be fully covered by single dye molecules due
to their large molecular size. Tai et al. [
22
] were able to solve this problem by
using a small molecule 3-phenylpropionic acid (PPA) as a co-modifier which
could cover the voids that were inaccessible for Ruthenium dye (see Fig.
9.16
) and
improved performance was achieved for the co-modified device due to the better
passivation of the backward recombination kinetics.
Search WWH ::

Custom Search