Environmental Engineering Reference
In-Depth Information
a less insulating material as compared to Al
2
O
3
, likely explaining the slow
degradation of the devices in air [
60
,
61
].
Kim et al. produced a TiO
x
layer by sol-gel procedure to modify the interface
of P3HT:PCBM and Al cathode [
62
]. This TiO
x
layer plays multiple functions as
electron-transporting layer, hole- and exciton-blocking layer, optical spacer, and
protecting layer during metal deposition. TiO
x
layer serves to passivate the devices
against intrusion of water vapor and oxygen, leading to remarkable enhancement
of operation lifetimes [
63
,
64
]. Moreover, the mechanism is proposed to be
ascribed to oxygen deficiencies in the TiO
x
film allowing adsorption sites for O
2
[
62
,
65
]. Lee et al. investigated the correlation between TiO
x
layer as optical
spacer and the processing additive 1,8-octanedithiol [
66
]. The results indicate that
the processing additive causes relatively rough surface of P3HT:PC
60
BM film and
reduces the effect as an optical spacer. Therefore, the dependence of processing
additive on surface roughness also influences the interface stability.
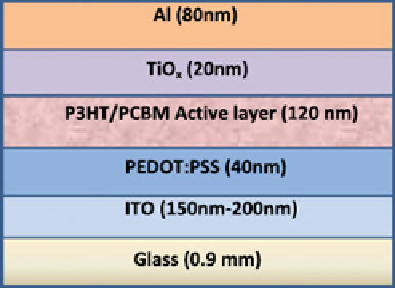
Li et al. reported a study of the stability of OPV under air and UV irradiation,
which consists of P3HT:PCBM as the active layer and 20 nm TiO
x
as the
protection layer prepared by partial hydrolysis of Ti-alkoxide in air [
35
], as shown
in Fig.
6.10
. It is stated that TiO
x
as the protection layer could make significant
improvement of device stability under air and UV exposure, implying a faster
decay due to UV exposure compared to air.
Figure
6.11
shows the dependence of main parameters of OPVs on UV
exposure time. It is clearly seen that the major loss in device efficiency under UV
irradiation is induced by the decrease in the J
sc
and the FF. Meanwhile, the V
oc
remains essentially constant. Similar results happen to the air-exposure conditions.
The measurements by transmission IR, UV spectroscopy, and ERS spectroscopy,
indicate that the sol-gel derived TiO
x
serves as an effective passivation film to
prevent oxygen when exposed to light due to the photo-oxidation of the bound
organic moieties causing oxygen gas scavenging [
35
]. The improvement mecha-
nism behind by using TiO
x
can be explained as [
35
]: the photochemical activity of
a TiO
x
film covers both Ti-OR functionalities and Ti-OH groups. From the results
of IR spectroscopy, it is found that the residual Ti-OR functionalities bound into
the sol-gel film are photo-oxidized, consuming O
2
(gas) and producing CO
2
(gas)
Fig. 6.10 OPV device
structure [
35
]. (Reprinted
from [
35
], with permission
from Elsevier) (
http://
Search WWH ::

Custom Search