Environmental Engineering Reference
In-Depth Information
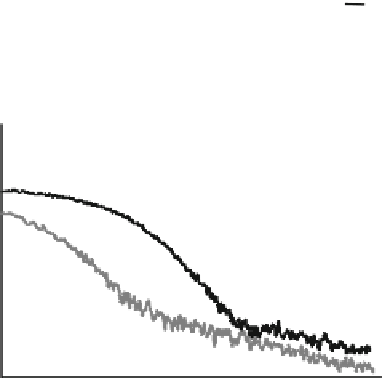
Fig. 5.8 a Transient
absorption spectra of
PT
10
PhT
10
:PCBM (95:5 w/w)
blend films excited at 420 nm
measured at 0, 1, 10, 100, and
3000 ps (from top to bottom).
b Transient absorption decays
of PT
10
PhT
10
:PCBM (95:5 w/
w) blend films excited at
420 nm monitored at 700 nm
under argon (black line) and
oxygen (gray line)
atmosphere. Reprinted with
permission from [
16
].
Copyright 2008 American
Chemical Society
(a)
0.15
0.10
0.05
0
600
700
800
900
1000
Wavelength / nm
(b)
10
-3
10
-4
10
-5
10
-6
10
-5
10
-4
Time / s
decay kinetics and the oxygen quenching measurement. Triplet excitons generally
decay monoexponentially while polarons typically exhibit the power-law decay
dynamics on a time scale of microseconds. In the presence of molecular oxygen, the
lifetime of triplet excitons is effectively shortened but the decay dynamics of
polarons does not change.
5.4.3 Other Charge Carriers
In order to assign charge carriers of unknown or new materials, it is necessary to
measure the absorption spectrum and to quantitatively evaluate the molar absorption
coefficient of each carrier separately using a model system with known donor or
acceptor materials. Here, we show an example of the assignment of PCBM anion by
using tetramethyl-p-phenylenediamine (TMPD) as a known electron donor.
Figure
5.9
shows transient absorption spectra of a polystyrene film doped with
TMPD and PCBM. Two absorption bands are observed at 570 and 1020 nm after the
laser excitation. The absorption band at 570 nm is in good agreement with that


























































































































Search WWH ::

Custom Search