Environmental Engineering Reference
In-Depth Information
Dekker and Wallis (1983) reported steam hydrolysis of sunflower seed hulls at 200°C for 5 min
followed by explosive defibration that solubilized more than 80% of the total hemicellulose and 85%
of the pectic substances. The remaining residue, which consisted of cellulose (38%), lignin (45%),
and residual hemicellulose (7%), was highly susceptible to hydrolysis by cellulases. Vaithanomsat
et al. (2009) also performed pretreatment of acid-soaked sunflower stalks by steam explosion at
207°C and 21 kg/cm
2
for 3 min to fractionate the cellulose, hemicellulose, and lignin. Ruiz et al.
(2006) used 220°C for 5 min as the pretreatment conditions for sunflower stalks. In our laboratory,
we used a low pressure of 1.05 kg/cm
2
for 30-90 min in an autoclave for NaOH -soaked sunflower
stalks and hulls (Sharma 2000; Sharma et al. 2002b). Similarly, Okur and Saracoglu (2006) used
relatively mild conditions of 0.7 M acid and 90°C for pretreatment of sunflower hulls.
Acid and alkali hydrolysis have been used for pretreatment of sunflower hulls and stalks. Whereas
Jimnez and Bonilla (1993), Vaithanomsat et al. (2009), and Okur and Saracoglu (2006) used acids,
Soto et al. (1994) treated the ground sunflower hulls with NaOH (0.5 to 3% w/w) in an autoclave at
120°C for 0.5, 1, and 1.5 h. They reported that the higher the NaOH concentration, the greater the
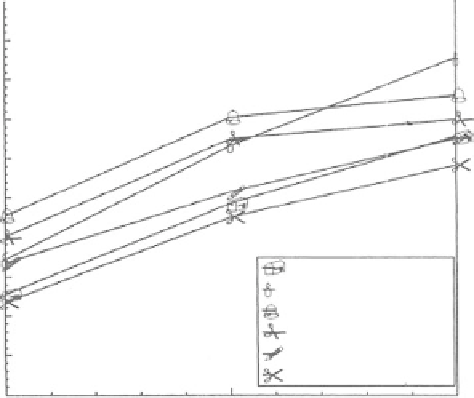
delignification. We also used alkali (0.25-1.5% NaOH; Figure 30.1) and standardized 0.5% NaOH
for pretreatment of sunflower stalks and hulls (Sharma et al. 2002b, 2004) that revealed 51 and 53%
cellulose, 17 and 17.5% hemicellulose, and 14.6 and 11.40% of lignin in sunflower stalks and hulls,
respectively (Sharma 2000; Sharma et al. 2002a). The extraction yield (fraction of sunflower stalks
recovered after pretreatment) was 66% (Sharma et al. 2002a).
30.5 cellulase ProductIon
Even a cursory perusal of current scientific literature shows cellulose hydrolysis by cellulases to be
among the most intensively studied topics. Each and every aspect of cellulase production such as
isolation and mutation of cellulolytic microorganisms, mode of fermentation, process optimization,
genetics and regulation at a molecular level, mode of action, process economics, and cellulase
recycling have been investigated comprehensively. Several workers have reviewed the cellulase
production and technology aspects (Saddler et al. 1986; Srinivasan and Seetalaxman 1988; Beguin
1990; Wyman 1994; Bothast and Saha 1997; Ward 2002; Juhasz et al. 2004; Immanuel et al. 2006;
Kocher et al. 2008).
200
190
180
170
160
150
140
0.25% (w/v) NaOH
130
0.50% (w/v) NaOH
0.75% (w/v) NaOH
120
1.00% (w/v) NaOH
1.25% (w/v) NaOH
1.50% (w/v) NaOH
110
100
0.5
1
1.5
Autoclaving time (hrs) at 1.05 kg/cm
2
FIGure 30.1
Effect of NaOH and autoclaving at 1.05 kg/cm
3
on the enzymatic hydrolysis of sunflower
stalks. Incubation time 48 h.