Environmental Engineering Reference
In-Depth Information
Fer
Lignin or GAX
O-
5
Ace
Ace
GlcA
Ace
GlcA
Ara
f
O-
3
O-
2
α
13
α
O-
3
α
12
12
β
β
β
β
β
β
β
β
β
β
β
β
β
---
Xyl yl
Xyl yl
Xyl yl
Xyl
Xyl
14
Xyl
14
Xyl
14
Xyl
Xyl
14
Xyl
14
Xyl --
14
14 14 14 14 14 14
14
α
α
13
12
O-
3
O-
3
O-
3
Ace
GlcA
Ace
Ace
Ara
f
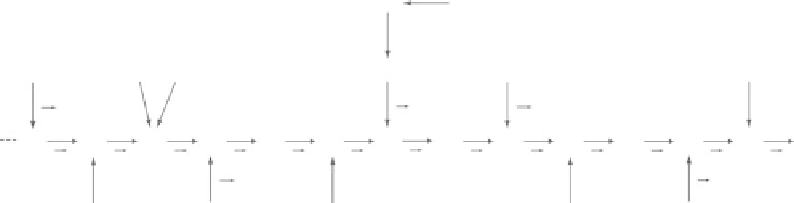
FIGure 16.9
Chemical structure of glucuronoarabinoxylan, a major wall constituent of grass cell wall.
Ace, acetate; Araf, arabinofuranose; Fer, ferulate; GlcA, glucuronate; Xyl, xylose. Arabinose/xylose ratio is
~0.1, glucuronate/xylose is ~0.2, and ferulate ester/arabinose is ~0.4 in a corn stalk (Jung and Casler 2006).
All of the arabinose and most of the glucuronate in stover are assumed to occur as GAX, with the remainder
of glucuronate present as potentially in other forms (e.g., trace amounts of pectin). Acetate concentration in
maize stover has been reported to be 30-50 g/kg of dry matter (Wooley et al. 1999; McAloon et al. 2000).
Assuming that all of it occurs in GAX and adjusting for molarity, approximately one-third to one-half of the
xylosyl residues on GAX are expected to be acetylated. (Adapted from Dhugga, K.S.,
Crop Sci
, 47, 2211-2227,
2007. With kind permission from the Crop Science Society of America.)
to the linkages found in the backbones of various hemicelluloses, it was postulated that cellulose
synthase-like (
Csl
) genes might be responsible for the biosynthesis of glycan backbones in the Golgi
(Richmond and Somerville 2000). Bioinformatics tools helped to identify
Csl
genes in various plant
species, which were then grouped into nine families,
CslA
through
CslH
and
CslJ
(Fincher 2009;
Van Erp and Walton 2009).
CslF
,
CslH
, and
CslJ
families are unique to the grasses, whereas
CslB
and
CslG
occur only in dicots. The remaining families are represented in both grasses and dicots.
Genes for the backbone formation of three of the hemicellulosic
polysaccharides—XG, MLG,
and β-(gluco)mannan—have been
identified (Dhugga et al. 2004; Liepman et al. 2005; Burton
et al. 2006; Cocuron et al. 2007; Doblin et al. 2009). The first successful identification of a Golgi
polysaccharide synthase was achieved through transcriptional profiling, whereby a
CslA
gene was
found to make β-1,4-mannan (Dhugga et al. 2004). Guar endosperm consists nearly entirely of
galactomannan, a hemicellulosic polysaccharide that is deposited in the cell wall after synthesis in
the Golgi apparatus and which serves the function of seed storage carbohydrates. In the expressed
sequence tag (EST) database of developing guar endosperm, a particular
CslA
gene was most
abundant at a developmental stage when the mannan synthase activity was at its peak. This
CslA
gene was identified and named mannan synthase (
ManS
) (Dhugga et al. 2004). Soybean somatic
embryos were used for functional characterization of
ManS
because soybean somatic embryos
do not incorporate significant amounts of mannose into polymeric form. Membrane particles
derived from the somatic embryos transformed with the
ManS
gene exhibited substantial mannan
synthase activity that was coincident with the level of expression of the gene, demonstrating that the
candidate gene indeed coded for mannan synthase (Dhugga et al. 2004). Some members of the
CslA
group from
Arabidopsis
were later found to possess (gluco)mannan synthase activity (Liepman
et al. 2005).
A similar approach was used to identify a synthase involved in the formation of glucan, which
forms the backbone of XG, from the nasturtium (
Tropaeolum majus
L.) seed EST database.
Nasturtium seeds accumulate XG as a storage polysaccharide (Cocuron et al. 2007). Expression
of the nasturtium
CslC
gene, which was the most abundant
Csl
gene in the EST database, in yeast
led the formation of β-1,4-glucan; however, co-expression of a xylosyltransferase did not produce
any XG (Cocuron et al. 2007). XG synthesis has been reported to require simultaneous actions
of glucan synthase and xylosyltransferase enzymes (Hayashi 1989; Faik et al. 2002). What then
could the role be of β-glucan produced by CslC? Purified Golgi fraction has been shown to make
β-1,4-glucan independent of xylose addition (Ray 1979). The enzyme catalyzing this reaction is