Environmental Engineering Reference
In-Depth Information
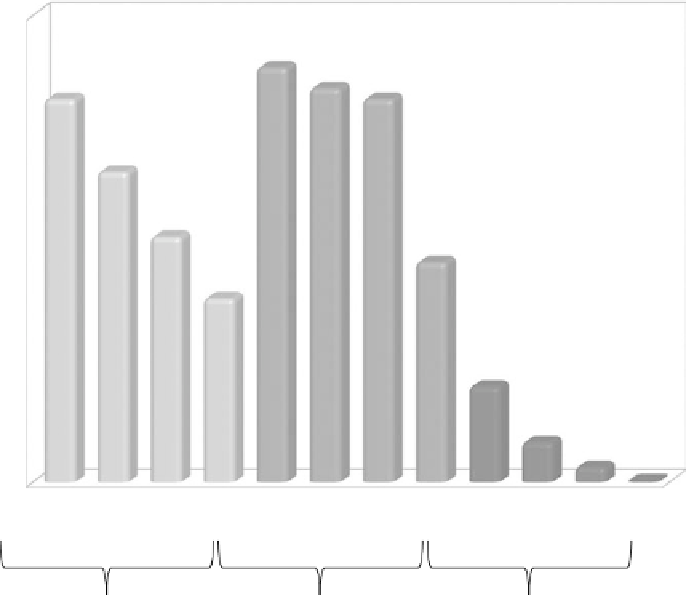
Hypothetical driving range for several fuels
900
Assumptions:
Vehicle is a typical mid-sized
sedan traveling at a constant
112 km/hr on a straight flat road
799
759
800
738
738
700
Overall fuel conversion
efficiency remains constant
among all fuels
598
600
472
500
424
354
400
300
184
200
77
100
29
1
0
Typically SI; liquid fuels
Typically CI; liquid fuels
Typically SI; gaseous fuels
(1)
Fisher-Tropsch (synthetic) diesel.
(2)
Soy-based methyl-ester biodiesel.
(3)
Dimethylether.
(4)
High-pressure methane. Pressurized to 25 MPa absolute @ 20°C and completely consumed during drive.
(5)
Pressurized to 34.5 MPa absolute @ 20°C and completely consumed during drive.
(6)
Wood-based (producer) syngas with typical constituent compositions from Borman and Ragland (1998).
(7)
Low-pressure methane. Barometric pressure (101.3 kPa) @ 20°C and completely consumed during drive.
FIGure 10.3
A hypothetical comparison of maximum driving range between fueling stops for different
types of fuels, assuming no changes to the vehicle, including constant fuel tank volume and constant overall
fuel conversion efficiency.
the same vehicle range (Komatsu et al. 2008). It is factors such as these that led to 93.6% of LDVs
sold in the United States in 2005 being designed to run on only gasoline or diesel. When adding to
this number hybrids and FFVs that use gasoline or diesel as one of the on-board energy sources,
the percentage increases to 99.96 (EIA 2007). However, with current increases in alternative energy
mandates, this percentage is likely to decrease in coming years.
10.1.3 f
uEl
E
conomy
and
co
2
r
EgulationS
It is through the reaction of fuel with air to produce CO
2
and water that chemical energy is converted
to sensible (thermal) energy that elevates the product gas temperature and enables a heat engine
such as the IC engine to extract energy from the working fluid. Therefore, CO
2
is an unavoidable
byproduct of combustion when fuel contains carbon, and for a given fuel, CO
2
production is directly
proportional to fuel consumption (every carbon atom in the fuel produces one CO
2
molecule). On
a mass basis, every kilogram of carbon (molecular weight of 12.01) in the fuel produces 3.67 kg of