Agriculture Reference
In-Depth Information
studies have shown that CLV3 protein is secreted and accumulates in the extracellular
space, where it might interact with the LRR domain of the CLV1/2 complex (Rojo
et al.
, 2002). These findings support a model in which CLV3 is secreted from
cells in the L1 and L2 layers and subsequently moves through the apoplast into
the underlying L3 layer where it binds to the extracellular domains of the putative
CLV1/2 receptor complex (stippled zone in Fig. 6.2A). According to this model,
increasing the concentration of ligand, and therefore activity of the CLV pathway,
should reduce the number of stem cells within the central zone, whereas less ligand
A
B
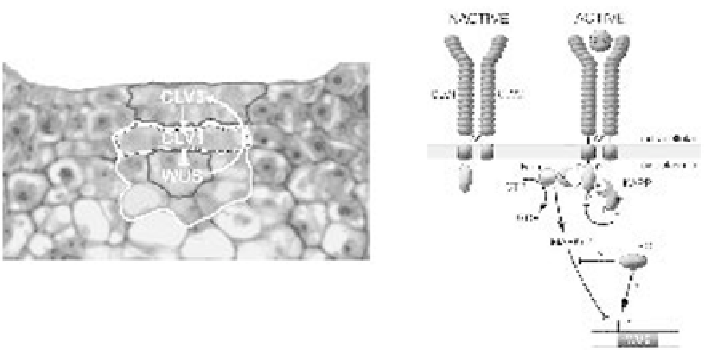
Figure 6.2
The
CLAVATA
signalling pathway. (A) Products of the three
CLAVATA
loci (
CLV1
,
CLV2
and
CLV3
) encode components of a signalling pathway that regulate the expression domain of the
homeodomain transcription factor
WUSCHEL
(
WUS
).
CLV3
is expressed predominantly in the L1 and
L2 cells of the central zone (black outline), whereas
CLV1
is expressed in L3 cells of the central and rib
zones (white outline).
WUS
expression is confined to just a few central zone cells underlying the stem
cells (black outline). These cells form an organising centre that promotes stem cell identity on the
overlying layers of the central zone through a non-cell autonomous signal. This signal also promotes
CLV3 expression, which subsequently activates the CLV1 signalling pathway (cells indicated with a
stippled outline), leading to repression of
WUS
expression. This negative feedback loop limits the size
of the stem cell population by regulating the size of the organising centre. (B) The molecular basis of
the CLV-WUS feedback loop. The receptor-like kinase CLV1 and receptor-like protein CLV2 are likely
to form an inactive 185-kDa heterodimer in the membrane of central zone cells via disulphide linkages
(S) between conserved cysteine pairs flanking the LRR. CLV1 is also likely to form dimers with other
receptor-like proteins (see text). Interactions between the CLV3 ligand and the external LRR domain of
the heterodimer cause autophosphorylation of CLV1 and the subsequent recruitment of other
intracellular proteins. The activated 450-kDa complex is associated with a Rho-like GTPase (Rop),
which is likely to be involved in signal transduction. Rop may bind to the activated complex via a small
linker protein (indicated with a question mark). Another component of the active complex is the protein
phosphatase (KAPP) that is likely to modulate CLV1 activity. Currently the downstream components of
the
CLV
signalling pathway are not known but it seems likely that a MAPK signalling cascade might be
involved. The role of the phosphatase 2C POLTERGEIST (POL) in the CLV signalling pathway is
ambiguous (see Table 6.2). It may act as a negative regulator of CLV signalling pathway, or as a
positive regulator of
WUS
. Redrawn from Carlos and Fletcher (2003).