Environmental Engineering Reference
In-Depth Information
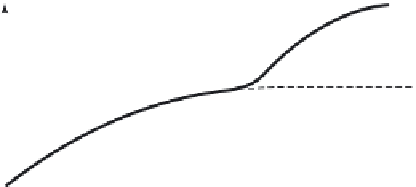
(CBOD), illustrated in Figure 2.4b, can be approxi-
mated by the following first-order model:
ultimate carbonaceous BOD
NBOD
BOD
u
dL
dt
5-day BOD
= −
k L
(2.9)
1
nitrification
begins
BOD
5
CBOD
where
L
is the CBOD (Ml
−3
) remaining at time
t
(T)
and
k
1
is a rate constant (T
−1
). If
L
0
is the CBOD remain-
ing at time
t
= 0, equal to the
ultimate CBOD
, Equation
(2.9) can be solved to yield
0
5
Time (days)
(a)
L L e
k t
=
−
1
BOD
u
(2.10)
0
L
Since the CBOD at time
t
is related to
L
by
L
0
CBOD =
L L
0
(2.11)
0
Time (days)
(b)
the CBOD as a function of time is given by combining
Equations (2.10) and (2.11) to yield
Figure 2.4.
(a) Typical BOD curve; (b) carbonaceous BOD
remaining versus time.
CBOD =
L
(
1
−
e
k t
−
)
(2.12)
1
0
The CBOD is exerted by heterotrophic organisms
that derive their energy for oxidation from an
organic carbon substrate, and NBOD is exerted by nitri-
fying bacteria that oxidize nitrogenous compounds in
the wastewater. The carbonaceous demand is usually
exerted first, with a lag in the growth of nitrifying bac-
teria. Normally, nitrogenous oxidation of raw sewage is
only important after 8-10 days of oxidation in the pres-
ence of excess oxygen; for treated sewage, however,
nitrification may be important after 1-2 days, due to the
large number of nitrifying bacteria typically found in
treated sewage (Tebbutt, 1998).
BOD tests are conducted using 300-ml glass bottles
in which a small sample of polluted water is mixed with
(clean) oxygen-saturated water containing a phosphate
buffer and inorganic nutrients. The mixture is incubated
in a stoppered bottle in the dark at 20°C, and the dis-
solved oxygen in the mixture is measured as a function
of time, usually for a minimum of 5 days. Since the
sample is incubated in the dark, there is no possibility
for photosynthesis to occur, so the oxygen concentra-
tion must either remain constant or decline. Since both
biological and chemical processes may cause a decline
in oxygen concentration, BOD should be understood to
refer to biochemical oxygen demand rather that simply
biological oxygen demand. If a problem with nitrifica-
tion is suspected in the BOD test, a specific nitrification
inhibitor can be added to the water sample so that only
the carbonaceous BOD is measured.
The cumulative oxygen demand of the polluted water
after 5 days is called the
5-day BOD
, and is usually
written as BOD
5
. The kinetics of carbonaceous BOD
The ultimate CBOD,
L
0
, can be expressed in terms of
the 5-day CBOD, CBOD
5
, as
CBOD
5
(2.13)
L
=
0
−
5
k
1
−
e
1
where both CBOD
5
and
k
1
are derived from the BOD
test data. The value of
k
1
depends on a number of factors,
such as the nature of the composition of the waste, the
ability of available microorganisms to degrade the waste,
and the temperature. For secondary-treated municipal
wastewaters,
k
1
is typically in the range 0.1-0.3 d
−1
at
20°C, which gives a
L
0
/CBOD
5
ratio of approximately
1.6. Schnoor, (1996) suggests a value of 1.47 for
L
0
/
CBOD
5
in municipal wastewater, and data reported by
lung (2001) indicate that 2.8 may be more typical for the
L
0
/CBOD
5
ratio. On average, biological oxidation is
complete in about 60-70 days for most domestic waste-
waters (lung, 2001), although little additional oxygen
depletion occurs after about 20 days (Vesilind and
Morgan, 2004). Municipal wastewater discharges with a
CBOD
5
less than or equal to 30 mg/l are typically con-
sidered acceptable, and it is recommended that commu-
nities discharging treated domestic wastewater into
lakes or pristine streams reduce their CBOD
5
to less
than 10 mg/l to protect the indigenous aquatic life
(Serrano, 1997).
It is interesting to note that the 5 days used in the
BOD measure was originally chosen as the standard
duration for expressing BOD because the BOD test
was devised by sanitary engineers in England where the























Search WWH ::

Custom Search