Environmental Engineering Reference
In-Depth Information
Step 2.
Conduct a soil sample analysis to determine
the fraction,
f
oc
, of organic carbon in the soil, the
bulk density,
ρ
b
, of the soil, the water-filled poros-
ity,
θ
w
, and the concentration,
c
s
, of organic com-
pound in the saturated soil (typically in mg/kg).
Step 3.
Calculate the theoretical pore water concen-
tration,
c
w
, assuming no nAPL, where
5.7 MONITORING WELLS
monitoring wells are essential components of all sub-
surface investigations involving the saturated zone, and
the proper design of monitoring wells is a prerequisite
for obtaining representative measurements of ground-
water quality. A typical monitoring well is illustrated in
Figure 5.12. monitoring wells basically consist of a per-
forated pipe (called the screen or intake) attached to a
solid pipe (called the
casing
or
riser
) installed in a drilled
borehole. The annular region between the well and the
borehole is filled with sand or gravel (called the gravel
pack) up to a level just above the screen, capped with a
sealant, and backfilled with grout to the ground surface.
General specifications for monitoring wells are given
below.
c
f K
ρ
s b
c
=
(5.84)
w
ρ
+
θ
b oc
oc
w
Step 4.
Compare
c
w
with the effective solubility,
c
0
,
such that if
c
w
>
c
0
, the presence of residual nAPL
is indicated.
EXAMPLE 5.17
Drilling.
monitoring wells in unconsolidated materi-
als are frequently installed using
hollow-stem
augers
, which are rotated into the ground to bring
up soil and create the boring. A typical hollow-
Analysis of a soil sample indicates 150 mg/kg benzene,
2% organic carbon in the soil matrix, a bulk density of
1800 kg/m
3
, and a water-filled porosity of 0.29. If the
benzene in the soil is a result of a gasoline spill, where
the gasoline contains a mole fraction of 1.5% benzene,
determine whether gasoline is present as an nAPL in
the soil. In accordance with Raoult's law, the maximum
concentration of benzene in groundwater can be taken
as the mole fraction of benzene in gasoline multiplied
by the solubility of pure benzene in water.
Well
identification
labeled
Vented cap
Protective casing
Drain hole
Protective cover
Concrete pad
Solution
From the given data,
c
s
= 150 mg/kg = 1.5 × 10
−4
kg/kg,
f
oc
= 2% = 0.02,
ρ
b
= 1800 kg/m
3
, and
θ
w
= 0.29. Appen-
dix B.2 gives
K
oc
= 10
1.92
= 83.2 cm
3
/g = 0.0832 m
3
/kg,
and the solubility of pure benzene is 1780 mg/L. Since
the spilled gasoline contains 1.5% benzene, the effec-
tive solubility of benzene derived from gasoline is
0.015(1780) = 26.7 mg/L. According to Equation (5.84),
the theoretical pore water concentration,
c
w
, assuming
no nAPL, is given by
Casing
material
Dry bentonite
pellets
Grout
Riser material
Centralizers
c
f K
ρ
Grout
s b
c
w
=
ρ
+
θ
Secondary
filter pack
b oc
oc
w
1 5 10 1800
1800 0 02 0 0832
( .
×
−
4
)(
)
=
(
)( .
)( .
)
+
0
.
29
Bore hole
Bentonite
seal
8= mg/L
Secondary
filter pack
Since
c
w
exceeds the solubility of benzene in water
(= 26.7 mg/L), the presence of of gasoline nAPL is
strongly indicated.
As a rule of thumb, if the hydrocarbon concentration
in the soil matrix exceeds 10,000 mg/kg (1% of soil
mass), the sample probably contains some nAPL
(Cohen and mercer, 1993; Feenstra et al., 1991).
Centralizers
Primary
filter pack
Well screen
Plug
Figure 5.12.
Typical monitoring well.









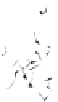























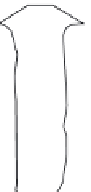















































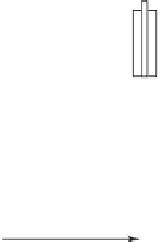









































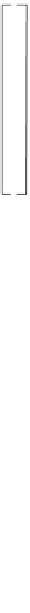




















































































































































































































































Search WWH ::

Custom Search