Environmental Engineering Reference
In-Depth Information
TABLE 5.12. Densities and Solubilities of NAPLs
solid
NAPL
Density
at 15°C
Solubility
at 10°C
air and/or water
Liquid
(kg/m
3
)
(mg/L)
LnAPLs
medium distillates (fuel oil)
820-860
3-8
Petroleum distillates (jet fuel)
770-830
10-150
Gasoline
720-780
150-300
Crude oil
800-880
3-25
DnAPLs
Trichloroethlene (TCE)
1460
1070
Tetrachloroethylene (PCE)
1620
160
1,1,1- Trichloroethane (TCA)
1320
1700
Dichloromethane (CH
2
Cl
2
)
1330
13,200
Chloroform (CHCl
3
)
1490
8200
Carbon tetrachloride (CCl
4
)
1590
785
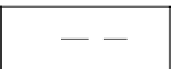
Figure 5.11.
Residual saturation in porous media.
Creosote
1110
20
Source of data
: Schnoor (1996).
impermeable boundaries are often difficult to locate
and remediate.
dissolution of soluble compounds and an associated
downstream plume. In some cases, the dissolved concen-
trations are sufficient to affect the density of the water
significantly, inducing a vertical groundwater velocity,
v
z
(LT
−1
), given by (Frind, 1982)
5.6.1 Residual Saturation
The movement of nAPLs in groundwater is governed
primarily by gravity, buoyancy, and capillary forces. At
low concentrations, nAPLs tend to become discontinu-
ous and immobilized by capillary forces, and they end
up trapped in the pores of aquifers, as illustrated in
Figure 5.11. In the vadose zone, the sorbed nAPL is
surrounded by both air and water, while in the saturated
zone, the sorbed nAPL is typically surrounded by
groundwater. The concentration of the sorbed nAPL is
termed the
residual saturation
, which is defined as the
fraction of total pore volume occupied by residual
nAPL under ambient groundwater flow conditions. In
the unsaturated zone, residual saturation values are
typically in the range of 5-20%, while in the saturated
zone, this range is typically higher and on the order of
15-50%. Residual saturation appears to be relatively
insensitive to the types of chemicals that comprise an
nAPL, but is very sensitive to soil properties and het-
erogeneities. The residual saturation,
S
r
, of nAPLs give
a good measure of how much of the contaminant will
remain trapped in the soil after the pure product has
percolated through the soil, and the residual saturation
is also a good measure of how much nAPL will remain
in the saturated zone after all the pure product is
pumped out of the aquifer. The residual saturation of
various petroleum fuels in soils are given in Table 5.13,
and the residual mass fraction,
M
f
(mm
−1
), can be cal-
culated using the relation
K
n
ρ
ρ
z
v
= −
−
1
(5.78)
z
e
o
where
K
z
is the vertical hydraulic conductivity of the
porous medium,
n
e
is the effective porosity (dimension-
less),
ρ
is the density of the dissolved mixture (mL
−3
),
and
ρ
o
is the density of the native groundwater (mL
−3
).
The relative magnitude of
v
z
to the horizontal seepage
velocity will give an indication of the extent to which
the contaminant plume moves in the same direction as
the groundwater flow.
Since LnAPLs do not penetrate very deeply into the
aquifer and are relatively biodegradable under natural
conditions, they are generally thought to be a more
manageable environmental problem than DnAPLs,
which tend to be trapped deep in the aquifer. Other
factors that make DnAPL contamination more diffi-
cult to remediate are: (1) Chlorinated solvents do not
biodegrade very rapidly and persist for long periods
of time in groundwater, in fact, products of microbial
degradation of halogenated solvents are sometimes
more toxic than the parent compounds; and (2) chlori-
nated solvents have physical properties, such as small
viscosities, that allow movement through very small
fractures and downward penetration to great distances.
The pattern of DnAPL penetration in aquifers is com-
monly referred to as
viscous fingering.
. DnAPL pools on
ρ
nS
n
f
r
M
=
(5.79)
f
ρ
(
1
−
)
+
ρ
nS
s
f
r


























































































Search WWH ::

Custom Search