Biomedical Engineering Reference
In-Depth Information
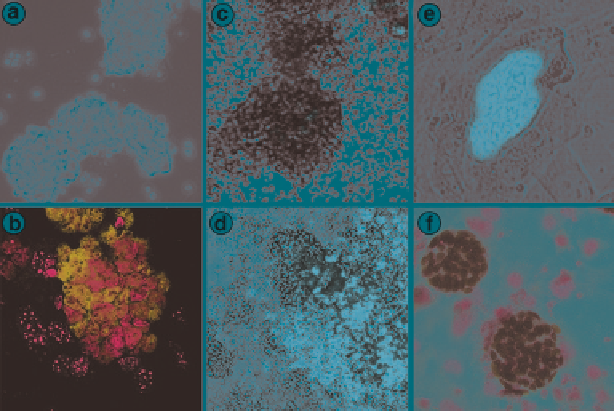
Fig. 2.3
Culture-induced up-regulation of OCT4 in spermatogonial stem and progenitor cells
coincident with appearance of ES-like colonies. SSCs were derived from adult wild-type or
OCT4-GFP reporter mice. (
a
) Phase contrast appearance of routine SSC cultures. (
b
) Specific
nuclear labeling of SSCs using anti-PLZF antibody (
red
). (
c
) Fluorescence microscopy for OCT4-
GFP reporter in routine SSC cultures. (
d
) Spontaneous up-regulation of OCT4-GFP (
green
) in
long-term culture of SSCs. (
e
) Uniform OCT4-GFP expression (
green
) in ES-like colonies
mechanically transferred to MEF feeder cells. (
f
) Immunohistochemistry demonstrating uniform
endogenous OCT4 protein expression (
brown
) in ES-like cells derived from SSC cultures.
Counterstain in (
b
) and (
f
) is
blue
(Seandel et al. unpublished data)
(3-8 weeks old) were the source of SSCs, no ES-like cells appeared, although the
parental adult SSC lines could be derived in only 20% of experiments. Adult-
derived
P53
knockout SSC, which could be derived at higher rate, also gave rise to
ES-like cells. The investigators provided substantial evidence that the ES-like cells
were not only distinct from the parental SSCs but that the ES-like cells could under-
take most if not all of the functions of ES cells, including long-term self-renewal in
culture, multi-lineage differentiation and formation of chimeric animals, including
germline transmission.
A major distinction from the parental SSCs was that the ES-like cells formed
teratomas in both subcutaneous teratoma assays and upon injection into the semi-
niferous tubules, indicating that the novel ES-like phenotype was stable and that the
cells could not simply revert back to the SSC phenotype upon placement back into
the normal SSC niche (see Table
2.1
) (Kanatsu-Shinohara et al.
2004
). This was in
contrast to the parental SSCs that did not form teratomas at all, consistent with our
own experience (Seandel et al. unpublished data). The authors proposed that a
predisposition to pluripotency could be a general property of SSCs but that the
somatic cells
in vivo
may help to suppress such aberrant cell phenotypes, in order
to prevent teratoma formation in the normal testis. Furthermore, the mechanism of
conversion of SSCs into ES-like cells appeared to be different from that in which
Search WWH ::

Custom Search