Environmental Engineering Reference
In-Depth Information
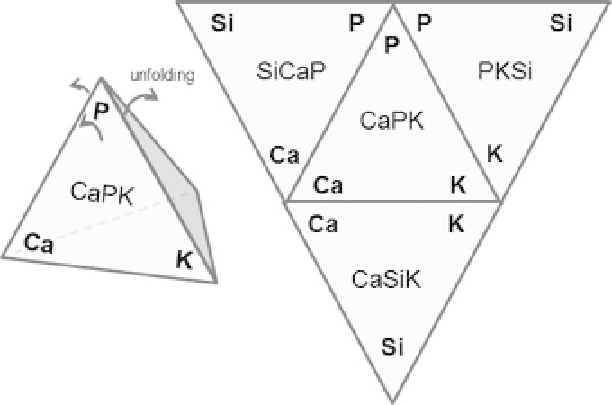
Figure 2.5. Ternary diagram for the elements Ca, P, K and Si in biomass, depicted as the unfolding of a
three-dimensional tetrahedron into two dimensions.
greatest contributions to this trend. When all of the woody species were treated as a single group,
the variation in their ash composition was greater than that for the herbaceous group.
According to Vassilev
et al
. (2010), the most abundant ash elements (in decreasing order) in
biomass are Ca, K, Si, Mg, Al, S, Fe, P, Cl, Na, Mn and Ti. In the earth's crust, their abundance
decreases in the following order: Si
>
Al
>
Na
>
K
>
Ca
>
Fe
>
Mg
>
Ti
>
P
>
Mn
>
S
>
Cl. All of these
species are rock-forming elements. Consequently, plants are enriched in Ca, K, Mg, S, and P and
have comparatively low levels of Si, Al, Fe and Ti relative to their abundance in the earth's crust.
It is possible that these 12 most abundant ash elements in biomass also account for 95% or more
of the total amount of mineral oxides that are formed during combustion and end up in the ashes.
Boström
et al
. (2012) have constructed a simplifiedmodel to describe the ash-forming reactions
by first considering the relative thermodynamic stabilities of the oxides of the different elements
involved in ash formation and then considering how they might react in the secondary reactions.
First, they reduced the number of significant ash elements to eight: Ca, P, Mg, S, K, Si, Na and
Cl, omitting Fe, Mn, Al and Ti. Fe and Mn were excluded because they often form individual
oxides that do not interact significantly with other ash elements during combustion. Al and Ti
were excluded because they are not essential metals for most plants, but certain soil conditions
and/or contamination can make them relatively abundant in ash; this is especially true for Al.
The oxides formed after the initial stages of combustion and the main oxides originating from
the organic components of biomass (i.e. H
2
O and CO
2
) are divided into two categories; basic
oxides and acid oxides; see Table 2.3 (after Boström
et al
., 2012). The thermodynamic stability
of all initial ash element oxides decreases with increasing temperature between 200 and 1600
◦
C.
However, the order of stability remains unchanged throughout this range, with a few exceptions.
The most notable exception is that the oxides of K and Na exhibit intermediate thermodynamic
stability at the lower end of this temperature range but are the least stable oxides of thosementioned
in Table 2.3 at the higher end of the range.
According to the model of Boström
et al
. (2012), Ca interacts with P, and two Ca phosphates
that are commonly found in woody biomass ashes were identified: apatite (Ca
5
(PO
4
)
3
OH) and
whitlockite (Ca
3
(PO
4
)
2
). If there is still an surplus of calcium oxide, the next most acidic oxide,
SO
2
(g) and/or SO
3
(g) will react to form Ca-sulfates. On the other hand, if there are P-based initial
oxides left over after all of theK, Na andCa oxides have been consumed, Mg-phosphate oxides will
be formed. The same is true for the other basic and acid oxides. While this is a simplified model,
it gives a good overview of how complex ash mixtures are formed during biomass combustion.
Search WWH ::

Custom Search