Environmental Engineering Reference
In-Depth Information
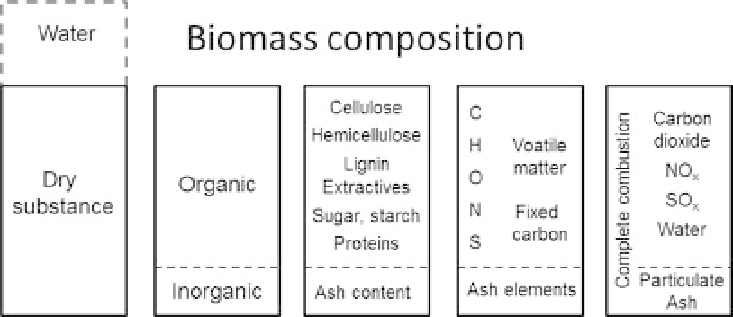
Figure 2.1. The different constituents of biomass.
Figure 2.2. Peak wavelength shifts around 1200 nm at different moisture contents (10-45% weight at wet
basis) and temperatures between -20 and 20
◦
C (from Lestander
et al
., 2008). The solid line is
the fiber saturation point and the dashed line is the freezing point of free water in the biomass.
During complete combustion, all of the elemental constituents of biomass are oxidized. This
generates gases, namely carbon dioxide, nitrogen (NO
x
) and sulfur (SO
x
) oxides, and water
(vapor), as well as solids in the form of fly ash, bottom ash, and particles of various sizes.
2.2.1
Water in biomass
While it may seem strange to treat water as a component of biomass, moisture content is a major
issue in most processes where biomass is used as a source of energy. The 'separation' of water
and dry substances in natural moist biomass, i.e. drying, is an energy-consuming process.
The nature of the moisture in biomass at different moisture contents and temperatures is illus-
trated in Figure 2.2, which shows the overtones of water molecule vibrations at one of three peaks
in the near-infrared region; the slower the molecular vibrations, the longer the wavelength (in the
z
-axis). The peak in the plot at high moisture contents for temperatures below 0
◦
C shows that
the overtones are shifted towards longer wavelengths under these conditions. This is due to the
formation of ice crystals within the biomass, which only occurs if its moisture content is above
the fiber saturation point. Below this point, water molecules are attached to binding sites on the
internal surfaces of the biomass and so the water molecules in this state do not undergo these
drastic changes with temperature. Notably, the 'floor' is somewhat tilted. Ongoing from higher

Search WWH ::

Custom Search