Environmental Engineering Reference
In-Depth Information
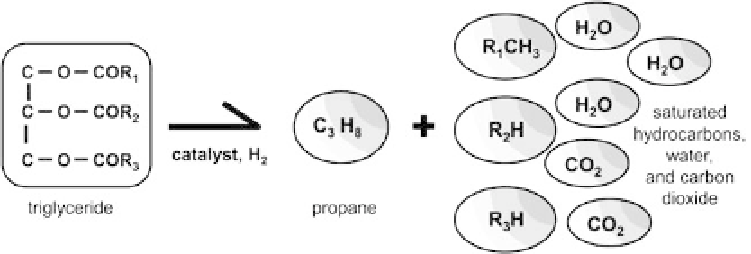
Figure 11.15. An example of hydrotreatment: deoxygenation of a triglyceride into saturated hydrocarbons,
water and carbon dioxide.
There are trade-offs to be made here. The isomerisation process is similar to the deoxygenation
reaction described above in that it operates at moderate temperatures (perhaps 250-350
◦
C) and
pressures (typically less than 5MPa). It uses excess H
2
and catalysts as reactants, and it will
produce the most jet fuel when the feedstock molecules are in the range of C10-C14. If the
feedstock has carbon chains that are either shorter or longer, the resulting hydrocarbon mix can
undergo fractional distillation to separate out the heavier and lighter hydrocarbons. The less
desirable components can be used for other purposes, such as green diesel or cooking fuel, but
it will supply less jet-grade fuel. Isomerisation is also more effective when the feedstock is not
fully saturated.
For the case of vegetable oil that is heavily weighted in the C16-C18 range and/or is fully
saturated, the more extreme hydrocracking option can be considered. At higher temperatures
(
350-420
◦
C), much higher pressures (7-14MPa), excess H
2
, and the right catalyst, the carbon-
to-carbon bonds of long-chained
n
-paraffins are ripped apart to form shorter
n
-paraffins and
branched isoparaffins. The resulting product will have a higher proportion of hydrocarbons in the
range needed for jet fuel, but, due to the large energy input, it will come at a cost, both in terms
of fuel cost and in environmental impact.
For a good reference on hydroprocessing, seeRobinson andDolbear (2006); on hydrogenization
of unsaturated methyl esters, see Bouriazos
et al.
(2010). Refining vegetable oils removes most of
the natural antioxidants such as tocopherols (Holser and Harry-O'Kuru, 2006). For a discussion
of additives and blending to improve cold-flow properties see Chastek (2011), Coutinho
et al.
(2010), Joshi
et al.
(2011), Kerschbaum and Rinke (2004), Kerschbaum
et al.
(2008), Moser
(2009), Wang
et al.
(2011) and emissions see Moser (2009).
∼
11.5.4
Other strategies
Another option for creating jet fuel is to start with relatively low-weight alcohols, such as butanol,
and perform an oligomerization step. In this reaction, the short-chained hydrocarbons undergo a
reaction to extend the length of the hydrocarbons, thus building up from C3 towards jet fuel range
hydrocarbons.
Some studies have examined the use of microbes to produce these compounds directly from
sunlight (Atsumi
et al.
, 2008; Tan
et al.
, 2011), fatty acid feedstocks (Dellomonaco
et al.
, 2010)
or through fermentation of sugars from lignocellulosic decomposition (Ha
et al.
, 2010). This
technology is still in the early stages and is not a near-term solution.
11.5.5
Co-products
One of the most critical aspects of sustainable process development and economic viability will
be the identification of value-added chemicals, energy, and materials from the remnants of the
Search WWH ::

Custom Search