Environmental Engineering Reference
In-Depth Information
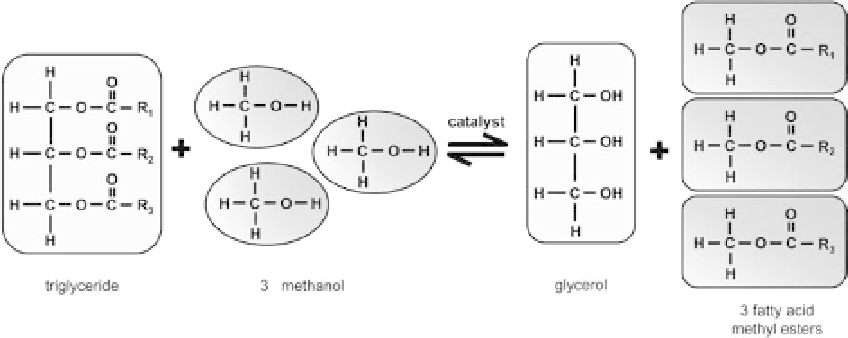
Figure 11.14. Transesterification is the reaction of a triglyceride and alcohol to form glycerol and alkyl
esters.
The process is reversible, in theory, but in actual practice, it is unlikely since the glycerol is not
miscible (i.e., it does not mix) with the product, although excess methanol can slow the separation.
The result is a two-phase system, with glycerol sedimenting to the bottom of the reaction vessel.
For biodesel, methanol is usually the alcohol of choice due to its low cost and ready availability,
although ethanol is used in places such as Brazil in which that fuel is abundant. Some studies
have examined the use of genetic modification of
E. coli
to produce higher chain alcohols, such
as isobutanol (Atsumi
et al.
, 2008), but this process is not yet ready for prime time.
A catalyst is a substance that speeds up, or otherwise assists, the speed or likelihood of a
chemical reaction, without itself being altered. The catalyst can be alkaline (such as sodium
hydroxide and sodiummethylate), acidic (such as sulfuric acid), or enzymatic. Strong acids donate
a proton to the carbonyl group, whereas bases remove a proton from the alcohol (Cordeiro
et al.
,
2011). Acidic catalysts usually provide the most complete reaction, although they are excessively
slower than alkaline catalysts, and they require higher temperatures. If free fatty acids are present
in the reactant (roughly
>
0.5-1%), reaction with alkaline catalysts will lead to a saponification
reaction, which turns the triglycerides into soaps rather than alkyl esters. In this case, a two-step
procedure is needed to use the quicker base catalysts. This method starts with a pretreatment step
with an acid catalyst which converts both free fatty acids and triglycerides to alkyl esters. After
this reaction is neutralized, it is followed by another conversion step with a base catalyst for the
remaining triglycerides. The additional energy and time requirements of acidic catalysis may be
more economical than the extra steps that are needed in a two-stage procedure for conversion
and catalyst separation. Lipase enzymatic catalysts are more expensive and usually require longer
reaction time but are more environmentally friendly. Alkaline protease was used in aqueous
enzymatic oil extraction for best results from jatropha (Achten
et al.
, 2008). The conversion of
palm oil from lipase enzymes produced by three bacterial strains that support transesterification
was tested, but it exhibited low conversion efficiency (
∼
20%
versus
commercial lipases at 90
+
%)
(Meng and Salihon, 2011).
After the reaction is complete, the FAAEs must be separated from the catalyst, excess alcohol,
water, free fatty acids, and the glycerol. The glycerol is relatively simple to divide from the
products, as it sediments to the bottom of the tank and does not mix with the product. For the
others, additional steps, such as distillation, would be needed.
Notice that, in Figure 11.14, the alkyl groups
R
1,
R
2 and
R
3 maintain their identities as
functional groups in the reactant and the product. Consequently, the fatty acid profile of biodiesel
corresponds in large measure to that of its feedstock. As seen in section 11.4.1, vegetable oils
are concentrated at higher carbon number (mostly at C16-C18), relative to petroleum jet fuel
(roughly C8-C16), as shown in section 11.3.1.
Search WWH ::

Custom Search