Environmental Engineering Reference
In-Depth Information
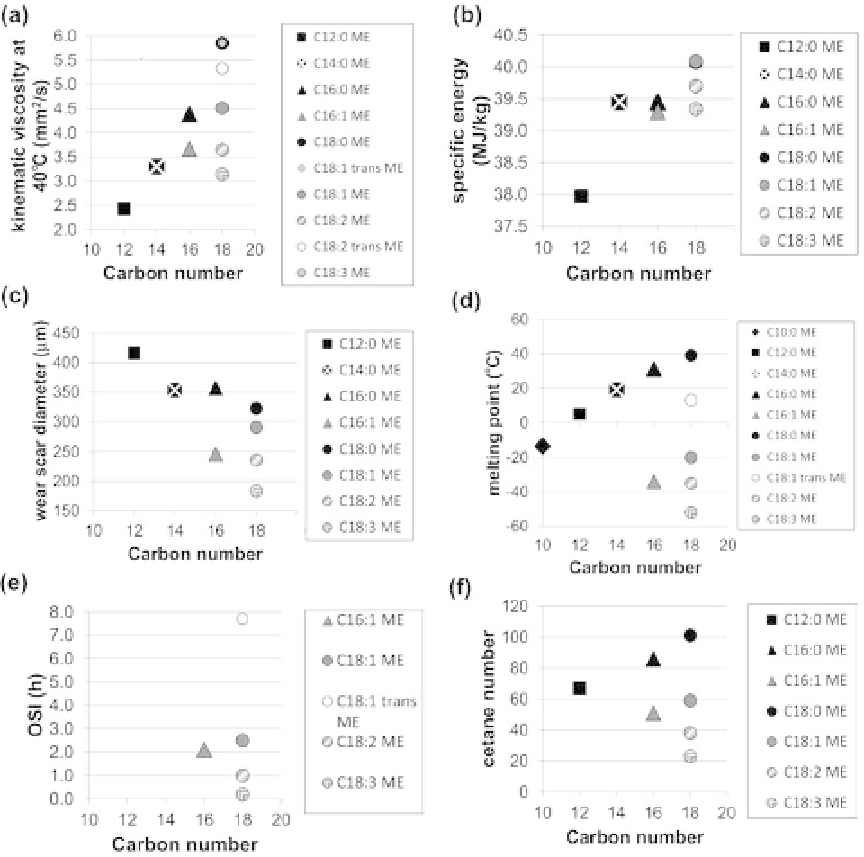
Figure 11.9. Dependence of the thermophysical properties of methyl esters (MEs) on carbon number and
saturation for (a) kinematic viscosity; (b) specific energy; (c) wear scar diameter, which is
a measure of lubricity; (d) melting point; (e) Oxidative Stability Index (OSI); and (f) cetane
number. Melting point for C10:0ME fromKnothe (2008a); remaining data fromMoser (2009).
The trends are different for other properties. For the specific energy (Fig. 11.9b), the value for
methyl oleate (C18:1) falls cleanly on top of methyl stearate (C18:0) at about 40MJ/kg, and the
value for methyl palmitoleate (C16:1) is nearly the same as that of methyl palmitate (C16:0).
Another feature in favor of methyl esters over ethyl esters for biofuel is that they exhibit greater
lubricity (Holser and Harry-O'Kuru, 2006). For lubricity (Fig. 11.9f), the wear scar diameter
decreases with increasing chain length (which indicates increasing lubricity). The lubricity also
increases with increasing saturation (i.e., wear scar diameter decreases for the C18:
y
MEs as
y
increases). At the molecular scale, oxygen-containing compounds like methyl esters can adsorb
or react on surfaces that rub together, reducing the friction between asperities (microscale or
smaller hills and valleys on a surface). This can reduce wear and the tendency toward seizure
(Fazal
et al.
, 2011). This is, however, highly dependent on maintaining a water-free environment
due to corrosive potential.
Search WWH ::

Custom Search