Environmental Engineering Reference
In-Depth Information
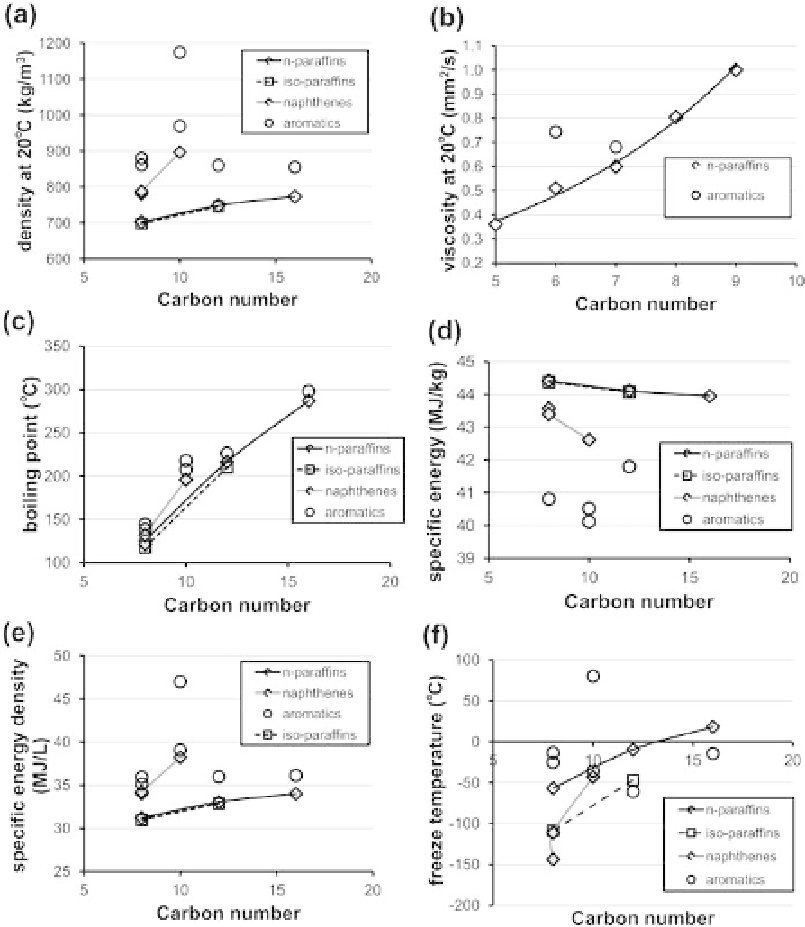
Figure 11.6. Compositional dependence of thermophysical properties of pure hydrocarbons. (a) Density
at 20
◦
C; (b) kinematic viscosity at 20
◦
C; (c) boiling point; (d) specific energy; (e) specific
energy density; and (f) freeze temperature. Data derived fromASTM (2011), Touloukian
et al
.
(1975) and TRC (2011).
be increased, even at the same carbon number, the fuel will have lower susceptibility to cold
temperatures, as well as a lower viscosity (Moses, 2008). The presence of naphthenes may deliver
better specific energy density with increasing carbon number from C8 to C10, but it comes at the
penalty of reduced tolerance to cold temperatures.
Other properties that are affected by carbon number include vapor pressure and lubricity. For
alkanes, vapor pressure increases with decreasing carbon number. Low-density hydrocarbons,
such as methane and propane, appear as vapors at standard pressure and temperature at ground
level (by convention, 101.325 kPa, 20
◦
C), while higher density hydrocarbons appear as liquids.
In general, increasing carbon number correlates to better lubricity, although that may also be
Search WWH ::

Custom Search