Biomedical Engineering Reference
In-Depth Information
unintentionally or provoke the release of reactive oxygen and nitrogen species that can interact
with DNA. They can even interact with DNA replication proteins or interfere with the DNA repair
mechanism. These interactions with DNA may lead to other responses such as mutagenesis and
carcinogenesis (Landsiedel et al. 2009; Singh et al. 2009). Nanogenotoxicology is the new area of
research that studies the deleterious effects of NPs on DNA. Nowadays, despite the high develop-
ment of NPs through medical or pharmaceutical applications, there are still very few studies regard-
ing their genotoxicity.
Genetic lesions can be produced at different levels: primary and secondary DNA structure (e.g.,
alteration of bases, cross-links, and adducts) or chromosomes (changes in number—aneugenic
effects—or structure—clastogenic effects) (Friedberg et al. 2006). Some of these lesions are always
produced by a direct interaction between a chemical compound and DNA (i.e., chemical adducts)
while others can also be produced by an indirect mode of action (e.g., producing ROS that interact
with the DNA, or decreasing the DNA repair capacity of the cell). Special attention is given to the
chemicals that produce mutations, since there is a clear relation between this type of lesion and
carcinogenesis.
Lesions at the level of the primary and secondary structures of DNA can easily be induced and
produce mutations if not repaired well, and so their presence can also be a risk factor for the occur-
rence of cancer. Numerical chromosomal changes have also been associated with tumorigenesis.
The importance of testing the genotoxicity of chemicals is obvious. In 2011, the International
Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for
Human Use (ICH) published a new version of the “Guidance on Genotoxicity Testing and Data
Interpretation for Pharmaceuticals Intended for Human Use” (ICH S2(R1) 2011), which has been
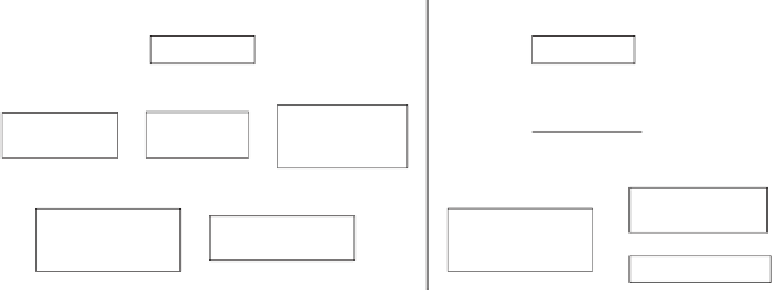
recently implemented. This guidance suggests a strategy to determine the genotoxicity of pharma-
ceutical compounds (Figure 17.1) and the use of OECD (Organisation for Economic Co-operation
and Development) guidelines to carry out different assays. The OECD guidelines cover the test-
ing of chemicals, including different protocols to carry out
in vitro
and
in vivo
genotoxicity tests
(http://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-
4-health-effects_20745788). Not all of the genotoxicity assays included in the ICH's strategy have
an OECD guideline at the moment, but efforts are being made to solve this gap and for the
in vivo
comet assay a draft guideline already exists.
Until now, there have been no regulatory requirements to test the genetic safety of NPs and it
is not clear if current strategies and standard assays for testing chemicals, ICH S2(R1) and OECD
guidelines, are suitable to characterize the potential genotoxic risk of NPs. A lot of effort is being
concentrated in developing a strategy and a battery of assays to check the genotoxicity of these
materials in a reliable way.
Strategy 1
Strategy 2
Ames test
Ames test
Mouse lymphoma
Tk
gene mutation
assay
Chromosome
aberration test
Micronucleus
test
or
or
In vitro
Alkaline elution
assay in liver
Chromosome
aberration test in
hematopoietic cells
Chromosome
aberration test in
hematopoietic cells
In vivo
(rodents)
Micronucleus test in
hematopoietic cells
or
and
or
Comet assay in liver
FIGURE 17.1
ICH's strategy for genotoxicity testing of pharmaceutical compounds.