Biomedical Engineering Reference
In-Depth Information
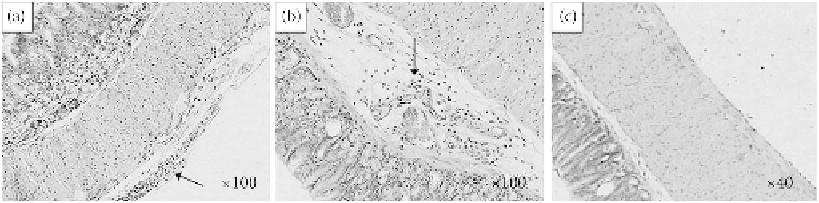
FIGURE 13.5
Histopathology of the stomach tissue (100× for A and B; 40× for C) in female mice 2 weeks
postexposure to different sized TiO
2
particles by a single oral administration of (a) control group (only exposure
to 0.5% HPMC), (b) 80 nm group, and (c) fine group. Arrows indicate the inflammation cells. (Reproduced
with permission from Wang, J. et al. 2007b.
Toxicol L ett
168:176 -185.)
as a central structural change, as the microvilli present on the apical surface of the epithelial sheets
are seminal to the cellular function of these intestinal cells. In addition, cytoplasmic signal trans-
duction underlies all changes that cells make over the short term to environmental stimuli, and
calcium signaling is a major form of signal transduction. They found that at 10 μg/mL and above,
TiO
2
NPs cross the epithelial lining of the intestinal model by transcytosis, albeit at low levels
(Koeneman et al. 2010).
A previous investigation reports that TiO
2
accumulates in the intestine of fish (Zhang et al. 2007)
and rats (Jani et al. 1994) where TiO
2
is translocated to systemic organs throughout the body.
Wang et al. evaluated the toxicity of fine and nanoparticulate TiO
2
: The acute toxicity of
nanosized TiO
2
particles (25 and 80 nm) on adult mice and compared it with fine TiO
2
particles
(155 nm). The histological photomicrographs of the stomach sections are shown in Figure 13.5a-c.
As depicted from the photomicrograph, there were some inflammatory cells in the chorion layer of
the stomach in mice of the 80 nm group (Figure 13.5), which was ascribed to the overload of par-
ticles in the stomach after a single oral administration of TiO
2
particles. In one of the control mice,
inflammatory cells in the mucosal layer of the stomach were also observed (Figure 13.5a), but it was
not representative. This effect may have been induced by the immune self-deficiency of this mouse
(Wang et al. 2007b).
13.5.2 t
oxIcIty
of
N
aNoscale
z
INc
p
oWder
Wang et al. evaluated the acute toxicity in the oral exposure to nanoscale zinc powder in mice.
The adult male and female healthy mice were gastrointestinally administered a dose of 5 g/kg
body weight with two particle sizes, nanoscale zinc (N-Zn) and microscale zinc (M-Zn) powder;
one group of mice was used as the control and treated with sodium carboxymethyl cellulose. The
symptoms and mortality after zinc powder treatment were recorded. The effects of different sized
particles on the blood element, blood coagulation, and serum biochemical levels were studied after
2 weeks of administration. Organs were collected for the histopathological examination. The N-Zn-
treated mice showed more severe symptoms of vomiting, diarrhea, and lethargy in the beginning
days than the M-Zn mice. Furthermore, during the initial 3 days, a 22% reduction in the weight
gain of mice exposed to NPs was observed when compared to the control group. The deaths of two
mice occurred in the N-Zn group after the first week of treatment. The mortalities after zinc powder
treatment were confirmed by the intestinal obstruction of N-Zn aggregations. The histopathologi-
cal examination found slight stomach and intestinal inflammation in almost all the nano and micro
Zn-administrated mice (Wang et al. 2006).
When mice were orally administered with 20 and 120 nm zinc oxide nanoparticles (ZnO
nanoparticles) at different doses, it was found that the damaged target organs showed different dose
response relationships. The 120 nm ZnO-treated mice had a positive dose-dependent pathological