Biomedical Engineering Reference
In-Depth Information
11.4.2 B
uckMINsterfullereNe
(c
60
f
ullereNe
)
A fullerene is a molecule composed completely of carbon and in the shape of a hollow sphere,
ellipsoid, or tube. At Rice University in 1985, spherical fullerenes, also called buckyballs or buck-
minsterfullerene (C
60
; Figure 11.5), were the first fullerene molecules to be discovered [79]. In 1996,
Sir Harry Kroto received the Nobel Prize in Chemistry for the discovery of buckyballs [80]. C
60
is a
truncated icosahedron containing 60 carbon atoms with C5-C5 single bonds forming pentagons and
C5-C6 double bonds forming hexagons. The diameter of a C
60
fullerene molecule is less than 1 nm
(average bond length ≈1.5 Å) and, hence, is an important member of the nanomaterial family [81].
They have been the subject of intense research, both for their unique chemistry and for their
technological applications, especially in materials science and nanotechnology.
They have captured the imagination of pharmaceutical researchers for their unique properties as
drug candidates. Derivatized fullerene can cross the cell membrane and bind to the mitochondria
as demonstrated by Foley et al. [82]. Moreover, DNA-functionalized fullerenes are able to enter
COS-1 cells and show comparable, or even better, efficiency than that of commercially available,
lipid-based vectors [83]. A lipophilic, slow-release, drug delivery system, which employs fullerene
derivatives to enhance therapeutic efficacy in tissue culture, was designed by Zakharian et al. [84].
Modified fullerenes have the potential to provide such a lipophilic, slow-release system, and are
composed of significant anticancer activities in cell culture as demonstrated with a C
60
-paclitaxel
conjugate.
Similarly, several biomedical uses have been explored using water-soluble C
60
-derivatives.
However, the employment of fullerenes for drug delivery is still at an early stage of development
primarily because of the associated toxicity issues. One study was performed to determine the
genotoxicity of fullerenes (a mixture of C
60
and C
70
) in a bacterial reverse mutation assay, including
the chromosomal aberration test, in hamster lung cells, followed by the acute, oral, median lethal
dose of fullerenes when applied to rats [85]. The results revealed that fullerenes did not have the
ability to induce acute oral toxicity or
in vitro
genotoxicity. Although water-soluble fullerenes are
not acutely toxic, they are retained in the organism for long periods, raising concerns about chronic
toxic effects [86]. Underivatized fullerenes aggregate in water where they are supposed to cause
oxidative damage to cellular membranes even at relatively low concentrations.
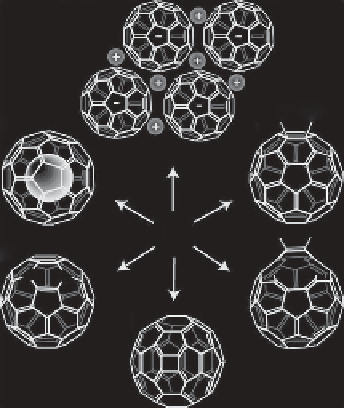
(a)
RR
(b)
(f )
RR
C
60
(c)
(e)
RR
N
N
(d)
FIGURE 11.5
Structure of fullerenes: (a) fullerene salts; (b) exohedral adducts; (c) open-cage fullerenes;
(d) quasi-fullerenes; (e) heterofullerenes; (f) endohedral fullerenes. (Adapted with permission from Hirsch A.
The era of carbon allotropes.
Nature Materials
. 2010;9:868-71.)