Environmental Engineering Reference
In-Depth Information
the long chain for the solvent provides the stabilisation of the dispersion. Generally,
aqueous dispersions of this type are insensitive to mild changes in pH and quite
large changes in ionic strength. Furthermore, the addition of polyvalent ions tends
to have little effect on the dispersion stability.
It is worth noting here the dynamics and properties of micelles and surfactants
such as sodium dodecylsulfate (SDS). These molecules form what are termed
association colloids. These are ordered aggregates of molecules which possess, for
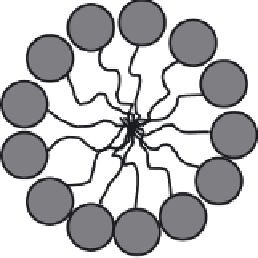
example, a hydrophobic core and a hydrophilic outer layer (Figure 2.10). The range
and properties of these systems and their phase behaviour are extremely complex.
However, their dynamics in stabilising a dispersion are important and the process
of forming micelles is key to the preparation of some polymer based nanoparticles.
The formation of a micelle, or the partitioning of a lyophillic surfactant at the
surface of a nanoparticle, is driven by the thermodynamics of the system. However,
the ability to form a stable dispersion depends on having suffi cient material in the
media. As a surfactant such as SDS is added to a solvent such as water it will seek
to minimise the interaction of the water with the hydrocarbon chain. At low con-
centrations this will initially result in a layer of the surfactant forming at the air-
water interface, thereby decreasing the surface tension of the liquid. As more
surfactant is added it will begin to form small (nm) aggregates called micelles which
present the charged head group to the aqueous media. Stabilisation of the micelle
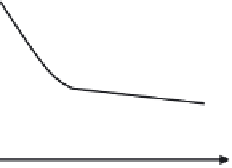
is therefore based on charge repulsion. The point at which the micelle may form is
called the critical micelle concentration (cmc) and represents the minimum amount
+
OSO
Na
3
Sodium dodecylsulphate
Hydrophobic hydrocarbon chain
Hydrophilic head
cmc
Surfactant concentration
Micelle
Figure 2.10
The parts of a surfactant (top), the structure of a micelle (bottom left) and the
rapid change in surface tension of a solution of the surfactant as the critical micelle concen-
tration (cmc) is exceeded.


Search WWH ::

Custom Search