Environmental Engineering Reference
In-Depth Information
Everything
Colloids
Molecules
4
1
2
3
8
5
7
6
Nanoparticles
Elements
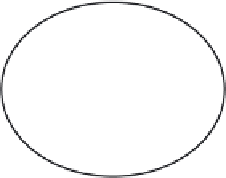
Figure 2.4
The interrelation of various types of material in the set of everything. Regions
1-8 relate to: 1 a stable colloid of an inorganic compound; 2 a stable colloid of an element;
3 a stable colloid of an element which has some molecular architecture; 4 a stable colloid
of a molecule; 5 an aggregate of an inorganic compound; 6 an aggregate of an element;
7 an aggregate of an element which has some molecular architecture; 8 an aggregate
of a molecule.
It is possible to describe nanoparticles as some subset of all material (Figure 2.4)
in which there are subsets of elements, molecules, colloids and nanoparticles. This
results in eight possible forms of nanoparticles, which can be divided into two sets;
those which form stable colloids (1-4) and those which do not (5-8). Examples of
these might be: 1 an inorganic compound (cadmium sulfi de); 2 a element (gold); 3
an element which has some molecular architecture (sulfur); 4 a molecule (polysty-
rene). Similar examples could be used to describe sets 5-8 but in this case these
materials do not form colloidal dispersions. These materials would therefore be
precipitated forms of 1-4. Examples of these might be nanoparticles deposited onto
a surface and thereby immobilised (titanium dioxide coated glass), or nanoparticles
which have formed massive aggregates. This clearly demonstrates the massive
diversity in what might be considered to be nanoparticle.
In addition to this, the shape of nanoparticles can be massively diverse. There is a
tendency to consider a particulate material to be isotropic, whereas a large range of
particle morphologies has been prepared. To date the most important nanoparticles
have proven to be the isotropic, the disc shaped and the wire or rod morphology.
However, a much wider range of morphologies are known, including tetrapod,
tear drop, dumbbell and dendrite structures (Figure 2.5) (Mana
et al.
, 2000 ;
Ung
et al.
, 2007 ).
Whilst there is massive diversity in composition and shape of nanoparticles their
architecture has common components which span a range of materials. These are
discussed below.


Search WWH ::

Custom Search