Environmental Engineering Reference
In-Depth Information
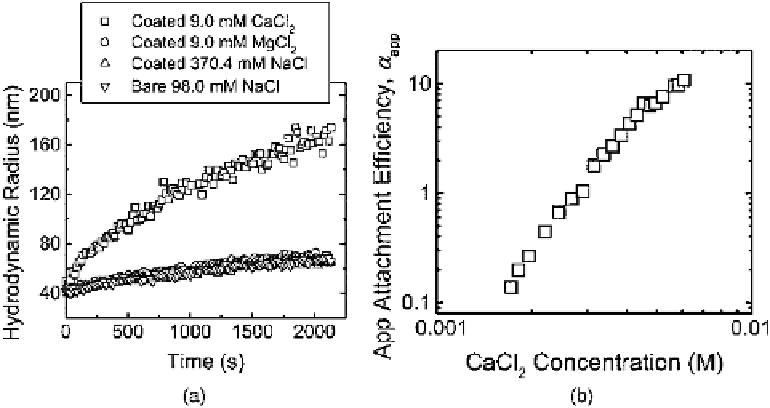
Figure 4.15
Enhanced aggregation of alginate-coated hematite nanoparticles in the pres-
ence of CaCl
2
. (a) Comparison of aggregation profi les of bare and alginate-coated hematite
nanoparticles in the presence of monovalent and divalent electrolytes. The bare hematite
and alginate-coated hematite nanoparticles are aggregated at pH 12.2 and 5.2, respectively.
For the four aggregation profi les, the particle concentration employed is 1.5
10
8
particles
per ml, and the unadsorbed alginate content for the alginate-coated hematite samples is
74.8 g/l. (b) Apparent attachment effi ciencies of alginate-coated hematite particles as a func-
tion of CaCl
2
concentration at pH 5.2. For all the aggregation experiments, the particle
concentration employed is 7.5
×
10
7
particles per ml, and the unadsorbed alginate content
is 37.4 g/l. (Reprinted from K.L. Chen, S.E. Mylon, M. Elimelech, Aggregation Kinetics of
Alginate-Coated Hematite Nanoparticles in Monovalent and Divalent Electrolytes,
Environmental Science & Technology
,
40
, 1516-23. Copyright 2006, American Chemical
Society.)
×
4.6.2
Disaggregation: Effect of Natural Organic Matter
Disaggregation is a very important process in determining colloidal fate and behav-
iour and interaction with trace contaminants, but few studies are available on the
disaggregation of natural colloidal particles. Recent work (Baalousha, 2008) inves-
tigated the role played by Suwannee River humic acid on the disaggregation of
synthesized iron oxide NPs. NOM has been shown to induce the disaggregation of
iron oxide aggregates (5- 10
m), likely due to formation of surface coating of NOM
on the surface and pore surfaces of aggregates and thus the enhancement of surface
charge as confi rmed by electrophoretic mobility measurements. This induces an
increase of the degree of repulsion within the aggregate matrix and results in
aggregate rupture. A previous study has shown that synthetic polymers are able
to separate two aggregated colloids, even when the separation distance was of
the order of few nanometres (primary minimum) (Ouali and Pefferkorn, 1994;
Pefferkorn, 1995 ).
There are two possible mechanisms of aggregate break-up based on aggregate
structure: surface erosion, which tends to be slow, and large scale fragmentation,
µ
Search WWH ::

Custom Search