Environmental Engineering Reference
In-Depth Information
coated with hydrous iron oxide to represent natural iron oxide particles. Figure
4.10b shows the interaction forces with the same system, but coated with NOM
(Suwannee river humic acid, SRHA) (Sander
et al.
, 2004 ). The adsorption of
humic acid to the surfaces results in signifi cant changes in the force- distance
relationship.
4.5.4.4
Polymer Bridging
Long chain organic molecules such as polysaccharides can attach to two or more
particles, thus bridging them together. Therefore, particles can form aggregates or
attach to surfaces even though they may be charged and repel each other, forming
open fl ocs. An example of the formation of such aggregates in a mixture of hae-
matite and alginate in the presence of 6.1 mM CaCl
2
is shown in Figure 4.11.
Direct measurements of bridging forces have been performed by AFM (Biggs
et al.
, 2000 ; Sander
et al.
, 2004 ). A distance -force relation ship measured by AFM
in the approach and separation force curves is shown in Figure 4.12. In the absence
of SRHA (Figure 4.12a), the approach and separation curves are identical. In the
presence of SRHA (Figure 4.12b), the separation curve shows a decrease in the
repulsive force for NaCl in comparison to the approach curve. However, in the
presence of CaCl
2
, the separation curve exhibits a signifi cant hysteresis with greater
attractive force close to the surface. This is explained by bridging attraction, that
is humic acid molecules function as an adhesive between the surfaces. More details
can be found elsewhere (Biggs, 1995; Dickinson and Eriksson, 1991; Grasso
et al.
,
2002 ).
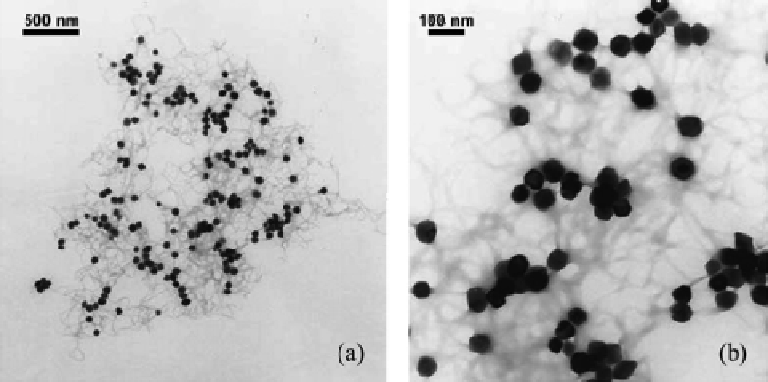
Figure 4.11
Combined hematite-alginate gel aggregate in the presence of 6.1 mM CaCl
2
at
pH 5.2. (a) and (b) are TEM images of the same aggregate, but at different magnifi cations.
(Reprinted from K.L. Chen, S.E. Mylon, M. Elimelech, Aggregation Kinetics of Alginate-Coated
Hematite Nanoparticles in Monovalent and Divalent Electrolytes,
Environmental Science &
Technology
,
40
, 1516-23. Copyright 2006, American Chemical Society.)
Search WWH ::

Custom Search