Environmental Engineering Reference
In-Depth Information
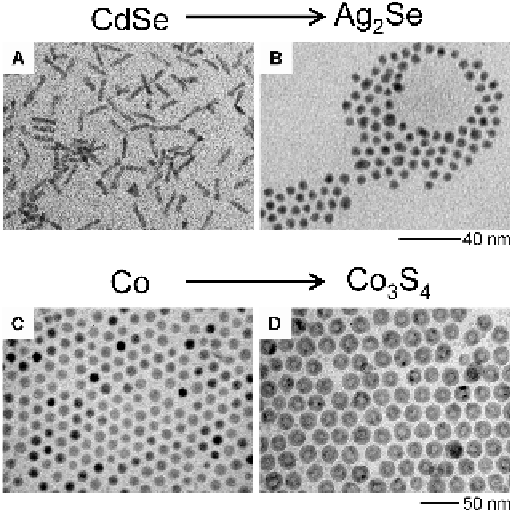
Figure 3.8
Examples of shape change after cation exchange reactions. A and B: Conversion
of nanorods to nanospheres when exchanging silver for cadmium. C and D: Conversion of
solid cobalt nanospheres to hollow cobalt sulfi de nanospheres, due to rapid diffusion of
cobalt ions. (A and B from Son
et al.
(2004)
Science
,
306
, 1009-12. C and D from Yin
et al
. (2004)
Science
,
304
, 711-4. Reprinted with permission from AAAS.)
fusion for a particular atom in a given solid lattice. Cation movement will not
necessarily occur for every sort of nanoparticle. Nevertheless, as nanoparticles will
doubtless encounter metal ions when they are released into natural systems, it is
important to keep these processes in mind.
3.6 Effect of Nanoparticle Aggregation on Physical and
Chemical Properties
Another structural characteristic that may well impact the behaviour and fate of
nanoparticles is their degree of aggregation. It is well established that under the
proper conditions nanoparticles can spontaneously self-assemble or aggregate
(Shipway
et al.
, 2000 ; He
et al.
, 2008 ; Guzman
et al.
, 2006 ; Gilbert
et al.
, 2007 ; Moreau
et al.
, 2007). Once such nanoparticles are released into chemically complex natural
systems, it is reasonable to expect that some of them will aggregate and that aggre-
gated nanoparticle systems may be as common as dispersed systems. Some exam-
ples of how aggregation may affect nanoparticle behaviour are briefl y discussed
here. The processes controlling aggregation are given in Chapter 4.
Search WWH ::

Custom Search