Environmental Engineering Reference
In-Depth Information
redox potentials in nanoparticles can also change other aspects of redox processes;
in cadmium sulfi de (CdS) nanoparticles, nitrate reduction rates were found to
increase with decreased particle size (Korgel and Monbouquette, 1997). As kinetic
phenomena can be important for many geochemical processes (Lasaga, 1998), such
effects may alter the geochemical impact of nanoparticles.
It should be emphasized that not every type of semiconductor nanoparticle dis-
plays quantum size effects. Their manifestation depends both upon the composition
of the nanoparticle and its size. Interestingly enough, even in the absence of
quantum size effects, nanoparticle size may affect the kinetics of redox processes.
For example, size can alter the average transit time for a charge carrier to diffuse
from a nanoparticle's interior to its surface (Hagfeldt and Gratzel, 1995).
The redox reactivity of an inorganic semiconductor nanoparticle is not only
determined by its core material but also by its coatings. Some coatings may enhance
charge carrier transport out of the nanoparticle. For example, an electroactive
ligand coating was developed for CdSe nanocrystals to improve their performance
in electronic devices, such as photovoltaic cells (Milliron
et al.
, 2003 ). Essentially,
the energy level alignment of the ligand coating molecules favoured the transfer
of charge carriers (holes) from the photoexcited CdSe nanoparticle to the ligand.
Adding a layer of zinc sufi de onto CdSe, on the other hand, can help to confi ne
charge carriers, because of the energetic positions of zinc sufi de band edges with
respect to those of CdSe (Dabbousi
et al.
, 1997 ; Hines and Guyot - Sionnest, 1996 ).
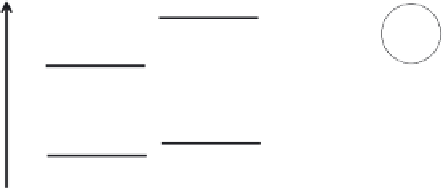
Energy level schematics for such systems are displayed in Figure 3.3. It is evident
(a)
(b)
ZnS
LUMO
e
-
Conduction
band edge
CdSe
HOMO
Valence
band edge
h
+
CdSe
CdSe
nanocrystal
ligand
ZnS
ZnS
Figure 3.3
(a) Schematic of valence and conduction band edges in a CdSe nanoparticle and
their energetic alignment with a molecular ligand coating the particle. Mobile charge carriers
(the hole and electron) are generated when a photon is absorbed by the nanoparticle, as
shown. Holes can be transferred to the highest occupied molecular orbital (HOMO) of the
ligand. (b) Schematic of a CdSe-ZnS core-shell nanoparticle and the corresponding band
edges of the core and the shell. ((a) Adapted from D.J. Milliron, A.P. Alivisatos, C. Pitois, C.
Edden and J.M.J. Frechet (2003) Electroactive Surfactant Designed to Mediate Electron
Transfer Between CdSe Nanocrystals and Organic Semiconductors,
Advanced Materials
,
15
,
58-61; copyright Wiley-VCH Verlag GmbH & Co. KGaA; reproduced with permission. (b)
Adapted with permission from B.O. Dabbousi, J. RodriguezViejo, F.V. Mikulec
et al.
(1997),
Journal of Physical Chemistry B
,
101
, 9463-75; copyright (1997) American Chemical Society.)




Search WWH ::

Custom Search