Environmental Engineering Reference
In-Depth Information
nomenon is provided by nano-sized molybdenum disulfi de (MoS
2
) (Abrams and
Wilcoxon, 2005; Thurston and Wilcoxon, 1999). The redox potential positions of the
valence and conduction bands of bulk and nanoparticulate molybdenum disulfi de
are shown in Figure 3.2. For comparison, the redox potentials of some environmen-
tally or biologically relevant half reactions are included. These reactions include
the generation of hydroxyl radicals (
•
OH) from water. Hydroxyl radicals can play
a major role in oxidative damage in biological systems (Imlay, 2003; Sayre
et al.
,
2008), as well as the degradation of organic compounds (Wilcoxon, 2000;
Kamat and Meisel, 2002). Also included are the reduction of AQDS (9, 10-
anthraquinone-2, 6-disulfonic acid) (Sund
et al.
, 2007), a synthetic analog of
electron-shuttling molecules used in bacterial respiration, and the reduction of
acetate to pyruvate (Becker and Deamer, 1986).
Notably, size effects in the photocatalytic activity of molybdenum disulfi de have
been demonstrated. It was shown that smaller molybdenum disulfi de nanoparticles
(4.5 nm and below) could photocatalyze redox reactions that would degrade organic
molecules, while larger nanoparticles (8-10 nm) could not (Abrams and Wilcoxon,
2005; Thurston and Wilcoxon, 1999; Wilcoxon, 2000). Results suggested that the size
dependence of photocatalytic activity was due to the higher redox potential of the
holes (Figure 3.2) in the smaller nanoparticles, which in turn could oxidize water
and create reactive hydroxyl radicals. This is a demonstration of how nanoparticle
size could infl uence the environmental or toxicological effects of a material. Altering
-1.0
Acetate/
pyruvate
AQDS
(electron shuttle)
0.0
MoS
2
(d=4.5 nm)
MoS
2
(bulk)
MoS
2
(d=8-10 nm)
1.0
H
2
O/
•
OH
2.0
Figure 3.2
Position of the conduction and valence band edges versus the normal hydrogen
electrode (NHE) for bulk and nanoparticulate MoS
2
, plus redox potentials for environmentally
or biologically relevant half reactions. Note that by varying size, the redox properties of MoS
2
are altered. For example, photoexcited 4.5 nm MoS
2
nanoparticles have holes with a redox
potential more positive than 1.2-1.5 V, which means these holes can oxidize water and create
hydroxyl radicals. The hydroxyl radicals can then degrade organic chemicals or, potentially,
cause oxidative damage in biological systems. Also displayed are redox potentials for the
conversion of acetate to pyruvate and for the reduction of AQDS, a synthetic analog of
electron-shuttling molecules important for bacterial respiration. (Adapted with permission
from T.R. Thurston and J.P. Wilcoxon (1999), Photooxidation of Organic Chemicals Catalyzed
by Nanoscale MoS
2
,
Journal of Physical Chemistry B
,
103
, 11-7. Copyright (1999) American
Chemical Society.)
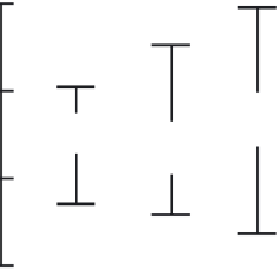



Search WWH ::

Custom Search