Environmental Engineering Reference
In-Depth Information
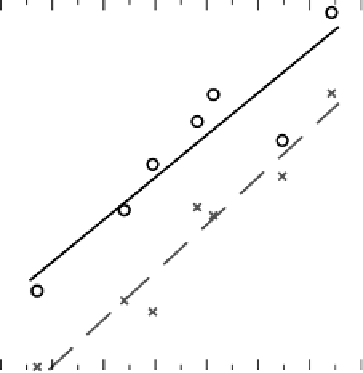
Figure 3.14 The full line and circles show the linear relation between E
OH
and E
O
for OH
adsorbed alone on the surface in the most stable site. The dashed line and crosses represent the
same relationship, but calculated for OH in the OH/H
2
O layer. The downward shift is related to
hydrogen bonding between water and OH. Another important effect is that the deviation from
the line is much smaller in the OH/H
2
O layer. The correlation coefficient is improved from
R
2
¼ 0.92 to 0.97 by including the water. This is partly because in the OH/H
2
O layer all
metals binds OH on top. The figure is based on data from [Karlberg, 2006].
note that this most likely is a good approximation. The results obtained with the water
bilayer compare reasonably well with the numbers obtained by this approach, meaning
that the bilayer is a robust model for water interaction.
3.5.2 Electric Field Effects
Apart from the water interaction, another interesting approximation in the model out-
lined above regards the electric field. In the electrochemical model described in this
chapter, the effect of the potential is taken into account by shifting the free energy
of states containing (H
þ
þ
e
2
)by2eU. However, since the electrode will be at a
different electric potential than the bulk of the conducting electrolyte, there will be
a potential drop over the electrolyte in the electrolyte region closest to the electrode.
This, in turn, means that there will be an electric field in this region. Whereas the
effect of this electric field on the binding energy of the relevant adsorbates can be esti-
mated to be small based on simple calculations [Nørskov et al., 2004], it could still be
interesting to look a bit more into this approximation.
In Fig. 3.15, the calculated shift in free energy of adsorption due to the electric field
is shown for various intermediates of interest for the ORR on Pt(111). The effect of the
electric field was taken into account explicitly by adding an external electric field to the



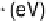

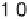






























Search WWH ::

Custom Search