Environmental Engineering Reference
In-Depth Information
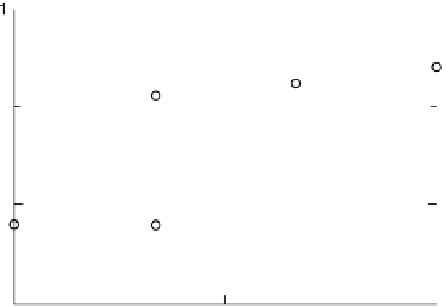
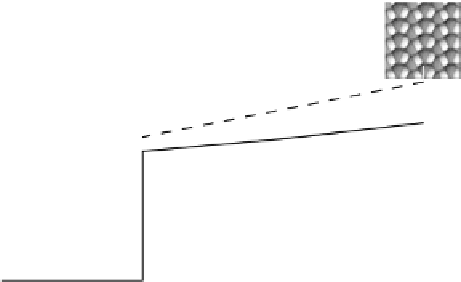
Figure 3.11 Estimation of the binding enthalpy of OH
as function of the coverage of OH
.
For coverages higher than
3
ML, the water 2OH interactions are locally replaced with OH 2OH
interactions. The energy of the latter interaction is estimated with a zigzag OH pattern on the
surface. It can be seen that the OH 2OH interaction is much smaller than the water 2OH
interaction.
In the following, we will take DG
OH
¼ 0.80 eV and 0.93 eV for Pt and P
3
Ni, respect-
ively, as was reported in [Stamenkovic et al., 2007a]. The resulting potential/coverage
curves are shown in Fig. 3.12, together with the experimentally obtained curves.
We conclude that the method seems to predict many of the features also seen in
well-controlled experiments even at a semiquantitative level.
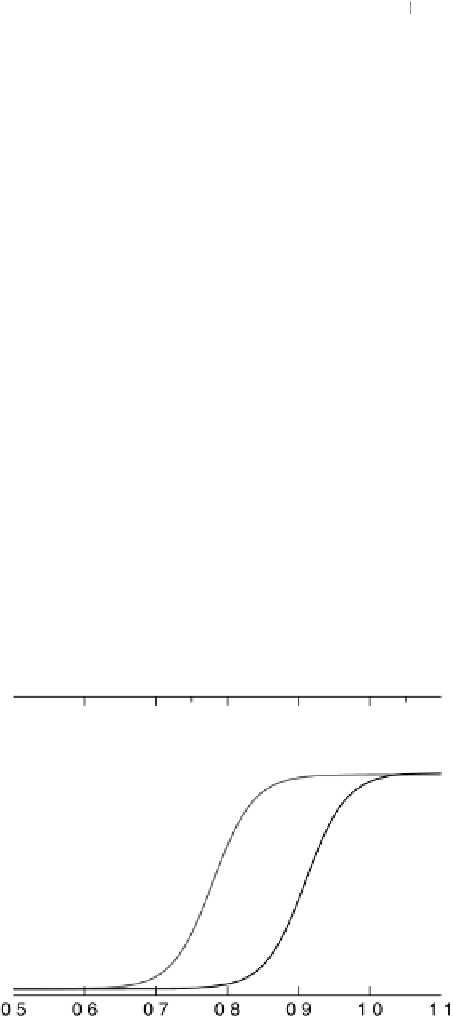
Figure 3.12 Coverage of OH
as function of the potential for Pt and Pt
3
Ni. Experimental
values from [Stamenkovic et al., 2007a] are represented by points connected with dashed lines.
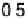
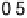
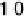






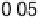
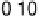

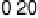
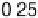

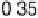


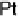
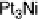
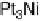










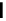


























Search WWH ::

Custom Search