Environmental Engineering Reference
In-Depth Information
Figure 3.10 Calculated current density based on Equation (3.5) (solid curves) and experimen-
tal current density from [Stamenkovic et al., 2007a] (dashed curves).
In the water/OH
layer, there is a perfect one-to-one ratio between water and
OH
, giving a coverage of
monolayer (ML) of OH
. Going beyond
3
ML of OH,
we have to replace a water22OH bond with an OH22OH bond. In the following, we
estimate the OH22OH interaction energy at a coverage of
1
3
1
1
3
ML of OH, without the
presence of water, and compare this energy with the corresponding result for
the OH/water layer (Fig. 3.11). Since the step is high, about 0.3 V, it is reasonable
to assume that the coverage never will be larger than
3
ML at potentials around U ¼
0.9 V. Although there is no consensus in the literature regarding the coverage of
OH during water splitting, we note that our maximum coverage of
1
3
ML is in good
agreement with the maximum coverage observed by [Stamenkovic et al., 2007a].
We include the configurational entropy of noninteracting particles, DS ¼ k
B
ln[(1 2 u
OH
)/u
OH
], for 0 , u
OH
,
3
. This expression assumes that OH molecules
do not interact, which we expect to be a good assumption, since all OH
only have
water as nearest neighbors as long as the coverage is smaller than
1
3
ML [Karlberg
and Wahnstrom, 2005]. We can therefore write the potential and coverage dependence
of the reaction free energy of Reaction (3.17) as
eU
1
u
OH
u
OH
DG(u
OH
, U)
¼
DG
OH
k
B
ln
(3
:
22)
1
Here DG
OH
is calculated for the standard condition of
3
ML OH and 1/3ML H
2
O.
Assuming that Reaction (3.18) is in equilibrium for all potentials [DG(U, u
OH
) ¼ 0],
and, furthermore, that the excess barrier for water splitting is small, this leads to the
following expression for the coverage:
u
OH
¼
1
3
1
1
þ
exp[(DG
OH
eU)
=
k
B
T]
(3
:
23)



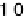


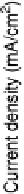







































Search WWH ::

Custom Search