Environmental Engineering Reference
In-Depth Information
Collman et al., 2007b]. However, when the electron delivery was slow, only the
bimetallic forms manifested ORR catalysis (it was presumed that the monometallic
Cu-free (Fe-only) catalysts degraded rapidly under these conditions) [Collman and
Boulatov, 2002; Collman et al., 2007b].
ORR catalysis by series 2 metalloporphyrins has been studied most extensively
[Boulatov et al., 2002; Collman et al., 2002a, 2003b; Shiryaeva et al., 2003], and
the results can be summarized as follows:
†
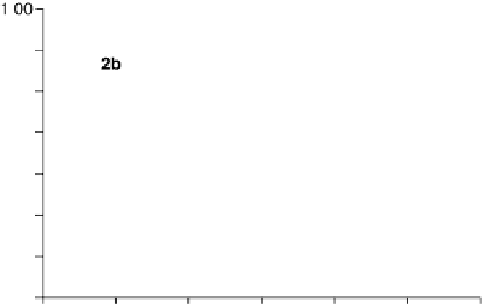
The Fe-only form of these metalloporphyrins is a highly selective ORR catalyst
when adsorbed on a graphite or Au electrode. It operates at an overpotential of
about 0.55 V at pH 7 and n
av
. 3.9 (Fig. 18.19) and retains these characteristics
for .10
4
turnovers; the catalytic selectivity is independent of the amount of
deposited catalyst.
†
Detectable amounts of partially reduced oxygen species are generated only
at potentials more oxidizing than 0.1 V (vs. NHE at pH 7); the primary product
was identified to be superoxide by incorporating into catalytic films selective
scavengers of H
2
O
2
,O
2
2
, and
OH. These catalysts do not appear to generate
significant amounts of
OH as do simple Fe porphyrins (see Section 18.4).
†
In contrast to simple metalloporphyrins, or cofacial diporphyrins, the catalytic
performance of these biomimetic catalysts improves at higher pH; as a result,
the smallest overpotential was observed at pH 8 (0.5 V) and at pH . 8no
partially reduced oxygen species could be detected at any potential.
†
These metalloporphyrins are unique among Fe and Co porphyrins in their high
catalytic efficiency of electroreduction of H
2
O
2
(at potentials ,0.75 V vs. NHE at
pH 7), as well as disproportionation and oxidation of H
2
O
2
(at potentials .0.8 V).
†
Despite the high catalytic activity toward H
2
O
2
reduction and disproportiona-
tion, ORR catalysis does not appear to proceed via free H
2
O
2
as inferred from
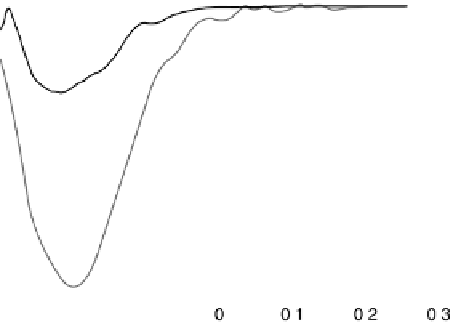
Figure 18.19 Selectivity toward four-electron oxygen reduction by graphite-adsorbed cata-
lysts 2b (Fig. 18.17) in the bimetallic (FeCu) and monometallic (Fe-only) forms at pH 7.



























Search WWH ::

Custom Search