Environmental Engineering Reference
In-Depth Information
2.7 SIMPLE INNER SPHERE ELECTRON TRANSFER
As demonstrated in Section 2.2, the energy of activation of simple electron transfer
reactions is determined by the energy of reorganization of the solvent, which is
typically about 0.5 - 1 eV. Thus, these reactions are typically much faster than bond-
breaking reactions, and do not require catalysis by a d-band. However, before consid-
ering the catalysis of bond breaking in detail, it is instructive to apply the ideas of the
preceding section to simple electron transfer, and see what effects the abandonment of
the wide band approximation has.
In inner sphere electron transfer, the interaction is so strong that the adiabatic theory
applies. Equations (2.6) - (2.8) remain valid, but, because of the more complicated
forms of the chemisorption functions D(1) and L(1), the electronic energy can no
longer be calculated analytically. However, numerical integration is easy, and poten-
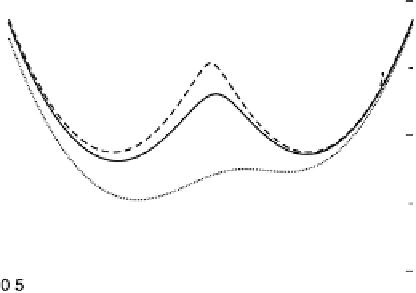
tial energy curves analogous to those shown in Fig. 2.4 can be calculated with ease. A
few examples are shown in Fig. 2.15. A coupling with a localized d-band can greatly
diminish the energy of activation—in extreme cases, it may even disappear [Santos
and Schmickler, 2007a, b, c]. Also, the coupling with a metal band not centered at
the Fermi level leads to a lack of symmetry between the forward and backward reac-
tions. The curves shown in Fig. 2.15 refer to equilibrium, even though the energies of
the initial states are somewhat lower than those for the final state. Equilibrium is deter-
mined by the situation in the bulk, where the electronic interactions are absent and
where, for the energy 1
a
¼
l chosen (see Section 2.2), the initial and final states
have the same energy.
In the activated state, the valence orbital passes the Fermi level of the metal, and the
electron transfer occurs. A d-band situated near the Fermi level will induce a strong
broadening of the reactant's density of states, or even to a splitting, as shown in
Figure 2.15 Potential energy curves for a simple electron transfer reaction at equilibrium.
The system parameters are l
¼
0
:
75 eV, w
0
¼
10 eV, D
sp
¼
0
:
05 eV, w
d
¼
1 eV,
and 1
c
¼
0
:
5 eV. Dashed line, D
d
¼ 0; full line, D
d
¼ 0.1 eV; dotted line, D
d
¼ 0.5 eV.


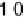
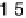
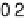
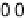
















Search WWH ::

Custom Search