Environmental Engineering Reference
In-Depth Information
Figure 18.17
Biomimetic ORR catalysts.
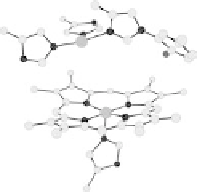
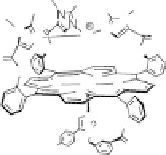
Section 18.2). The latest generation of such catalysts (1 in Fig. 18.17) reproduces the
key features of the site: (i) the proximal imidazole ligation of the heme; (ii) the trisi-
midazole ligation of distal Cu; (iii) the Fe - Cu separation; and (iv) the distal phenol
covalently attached to one of the imidazoles. As a result, binding of O
2
to compound
1 in its reduced (Fe
II
/Cu
I
) state appears to result in rapid reduction of O
2
to the level of
oxides (22 oxidation state) without the need for outer-sphere electron transfer steps
[Collman et al., 2007b]. This reactivity is analogous to that of the heme/Cu site of
cytochrome c oxidase (see Section 18.2).
Biomimetic studies typically have one or more of the following objectives: (i) to
reproduce in a small synthetic molecule reactivity that was theretofore only observed
in an enzyme; (ii) to understand the mechanisms of an enzymatic reaction and
the relationship between the stereoelectronic attributes of the catalytic site and its
reactivity; and (iii) to develop practical catalysts by exploiting and adopting solutions
that evolved in Nature. Biomimetic studies of cytochrome c oxidase have been
particularly impactfull in addressing aim (ii). On the other hand, this approach is









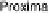










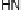
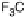




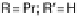

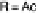













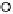








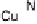
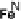











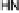





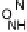













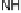








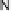




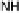








































Search WWH ::

Custom Search