Environmental Engineering Reference
In-Depth Information
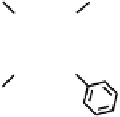
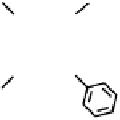
Figure 18.12
Chemical structures of selected simple Co porphyrins described in the text.
Co(Py
3
P), and tris-(4-cyanophenyl)-(N-methyl-4-pyridyl)porphyrin CoCP (Fig. 18.12),
in which the para-cyano groups were thought to coordinate to Ru
II
. Using
[Ru(edta)(OH
2
)]
2
as a source of Ru or using other porphyrins with coordinating
peripheral substituents (Fig. 18.12) did not produce catalysts superior to simple
Co porphyrins. However, exposing graphite-adsorbed Co(TPyP) to a solution of
[Os(NH
3
)
5
(OH
2
)]
2
þ
appears to have yielded an improved catalyst, and it was recently
reported that coordination of a single Pt complex to a Co porphyrin may also yield an
ORR catalyst with n
av
. 2 [Gadamsetti and Swavey, 2006]. The Co(TPyP) fragment
remains the most widely used porphyrin for studying the effect of peripheral metal
complexes on metalloporphyrin ORR catalysis [Araki and Toma, 2006].
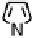

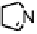
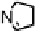
















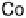

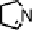
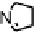



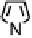


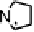




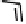
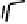
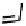




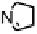

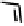








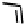




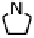
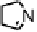

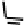

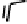
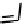

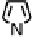






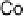







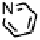












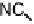


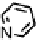
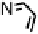



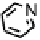










































































Search WWH ::

Custom Search