Environmental Engineering Reference
In-Depth Information
which may proceed through disproportionation of H
2
O
2
and reduction of the
resultant O
2
.
†
Depending on pH, increasing the acidity of the solution either makes the poten-
tial required to yield a fixed turnover frequency more oxidizing by 60 mV/pH or
does not affect it. This pH dependence is in most cases the same as that of the
Fe
III/II
couple in the absence of a substrate. These identical pH dependences
suggest a pre-equilibrium between the ferric and ferrous forms of the catalyst
followed, by a kinetically irreversible step that does not involve proton or
electron transfer (e.g., O
2
binding).
†
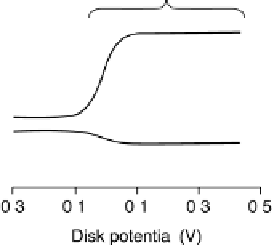
The apparent redox stoichiometry of O
2
reduction catalysis [n
av
, Reaction (18.8)]
is pH-independent, but for many catalysts depends strongly on the applied poten-
tial (Fig. 18.10). The apparent selectivity of Fe porphyrins deposited on the elec-
trode surface typically increases with the amount of deposited catalyst.
†
Usually, simple Fe porphyrins degrade rapidly during catalytic reduction of
O
2
or of H
2
O
2
.
Figure 18.10 Three types of polarization curves typically manifested by simple Fe and Co
porphyrins and cofacial metalloporphyrins (simulated voltammograms).
















































Search WWH ::

Custom Search