Environmental Engineering Reference
In-Depth Information
conditions using a catalyst whose n value is reliably established, for example graphite
(n ¼ 2) or thoroughly cleaned Pt (n ¼ 4, Fig. 18.7b). The latter method may provide
more precise estimates of n
av
, as the values of D, n, and [O
2
]
bulk
under given exper-
imental conditions are usually not known with sufficient precision (for example, the
literature values of D vary by a factor of 2 [Opekar and Beran, 1976] and solutions
oversaturated in O
2
are easily obtained if O
2
is continuously bubbled in).
Reliable estimates of k typically cannot be obtained from a single Koutecky -
Levich intercept, often referred to as i
k
21
, because of uncertainty in the amount of
catalytically active porphyrin at the electrode. To obtain reliable estimates of k,
Koutecky - Levich intercepts acquired at electrodes with varying surface coverage
of the catalyst, G
cat
, have to be plotted against G
cat
. It is typical to observe a direct pro-
portionality between i
k
21
and G
cat
at low surface coverages of the catalyst ( provided
that O
2
reduction at the electrode material does not interfere) that plateaus as the cover-
age increases and may even decrease with a further increase in coverage (Fig. 18.8),
signifying a change in the rate-determining step from catalytic reaction to mass or
charge transport in the catalytic film. Only at the catalyst coverages that yield intercepts
directly proportional to G
cat
can the Koutecky - Levich equation be applied.
One also needs to be careful when using the slope of the Koutecky - Levich plot to
determine n
av
of the catalytic film. Examples of metalloporphyrin-catalyzed ORR
have been reported where, above a certain value of the electrode rotational frequency,
the catalytic currents became independent of v, indicative of a breakdown of the
Koutecky - Levich model, either because the rate of charge or substrate transfer
within the film became rate-limiting or the catalyst became partially saturated with
O
2
[Boulatov et al., 2002; Song et al., 1998; Collman et al., 1980]. In other cases,
the i
1
cat
versus v
21/2
graphs may remain mostly linear within the experimental
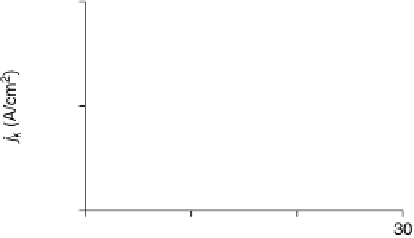
Figure 18.8 The inverse of the intercept of the Koutecky - Levich plots normalized to the
geometric area of the electrode, j
k
, versus surface coverage of two biomimetic Fe porphyrin cat-
alysts deposited on an edge-plane graphite electrode [Boulatov et al., 2002]. At surface cov-
erages ,1 - 2 nmol/cm
2
(depending on the catalyst), j
k
is directly proportional to G
cat
.
Depositing more catalyst leads to a drop in j
k
, suggesting a change in the turnover-determining
step. The surface coverages are per geometric area; because of the high roughness of the edge-
plane graphite, 1 - 2 nmol/cm
2
may correspond to only few monolayers of the catalyst.
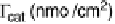











Search WWH ::

Custom Search