Environmental Engineering Reference
In-Depth Information
Figure 18.6 Energetics of the ORR at the heme/Cu site of CcO; the enzyme couples oxi-
dation of ferrocytochrome c (standard potential about 2250 mV; all potentials are listed with
respect to a normal hydrogen electrode) to reduction of O
2
(standard potential at pH 7 800
mV). Of the 550 mV difference, only 100 mV is dissipated to drive the reaction; 220 mV is
expanded to translocate four protons from the basic matrix compartment to the acidic IMS (inter-
membrane space). In addition 200 mV is converted into transmembrane electrostatic potential as
ferrocytochrome is oxidized in the IMS, but the charge-compensating protons are taken from the
matrix. The potentials are approximate.
18.3 EXPERIMENTAL APPROACHES TO STUDYING ORR
CATALYSIS BY SYNTHETIC METALLOPORPHYRINS
Studies of ORR catalysis—Reaction (18.8) below—by metalloporphyrins typically
aim at quantifying how selective the catalyst is towards the complete four-electron
reduction of O
2
versus partial reduction, typically two-electron reduction to H
2
O
2
.
Reduction of O
2
to O
2
2
(superoxide, or its conjugate acid, hydroperoxyl radical
HO
2
) may also occur, particularly with Fe porphyrins, which are susceptible to auto-
xidation [Shikama, 1998] (see Section 18.6.2), but this pathway is rarely considered.
This selectivity is often quantified as n
av
, the average number of electrons by which
one molecule of O
2
is reduced. It is related to a, the fraction of the four-electron path-
way, by n
av
¼ 2(1
þ
a) if reduction to H
2
O
2
is the only competing process. A fairly
small subset of reported studies have aimed at quantifying the kinetics of the reduction
in terms of the bimolecular rate constant k. By examining how n
av
and k depend on
experimental conditions ( potential of the electrons, H
þ
and O
2
concentrations, and
concentration or surface coverage of the catalyst) mechanistic inferences about
Reaction (18.8) can sometimes be made, but the required careful mechanistic studies
have only rarely been performed.
O
2
þ
2(1
þ
a)H
þ
2(1
þ
a)e
þ
catalyst
k
2aH
2
O
þ
(1
a)H
2
O
2
þ
catalyst
(18
:
8)
Most studies of ORR catalysis by metalloporphyrins have been carried out using
water-insoluble catalysts absorbed on a graphite electrode in contact with aqueous
solution. In a limited number of cases, four other approaches have been used: catalysts
imbedded in an inert film (i.e., Nafion or lipid) on the electrode surface; self-assembled
monolayers of catalysts; catalysts in aqueous or mixed organic/aqueous solutions in
contact with an electrode; and catalysis in mixed aqueous/organic medium using



















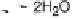
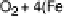




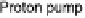



















































Search WWH ::

Custom Search