Environmental Engineering Reference
In-Depth Information
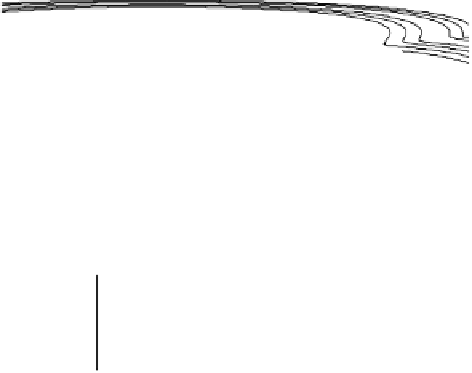
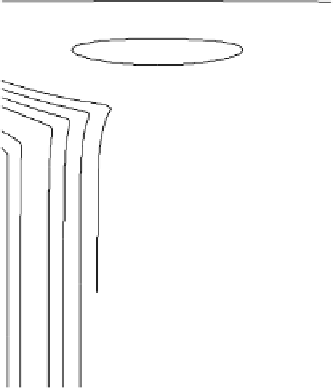
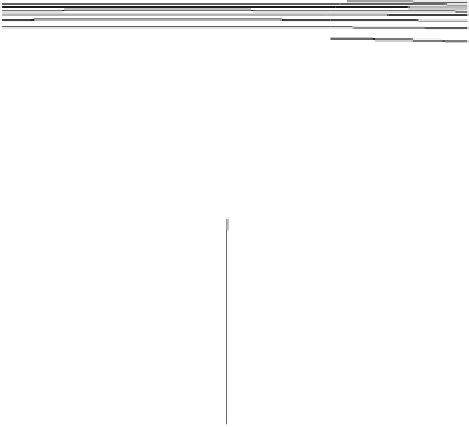
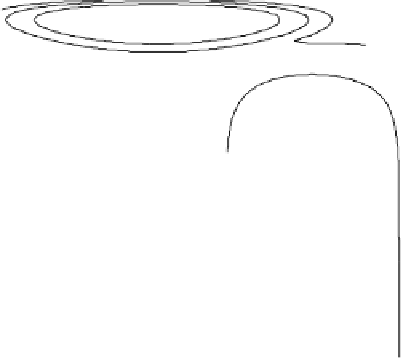
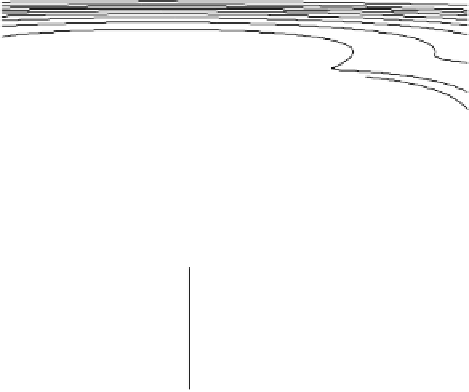
Figure 2.11 Potential energy surface for a simple bond-breaking reaction in Sav´ant's model
[Sav´ant, 1993].
2.6 INTERACTION OF A REACTANT ORBITAL WITH
A NARROW BAND
In the electron transfer theories discussed so far, the metal has been treated as a struc-
tureless donor or acceptor of electrons—its electronic structure has not been considered.
Mathematically, this view is expressed in the wide band approximation, in which D is
considered as independent of the electronic energy 1. For the sp-metals, which near the
Fermi level have just a wide, structureless band composed of s- and p-states, this
approximation is justified. However, these metals are generally bad catalysts; for
example, the hydrogen oxidation reaction proceeds very slowly on all sp-metals,
but rapidly on transition metals such as platinum and palladium [Trasatti, 1977].
Therefore, a theory of electrocatalysis must abandon the wide band approximation,
and take account of the details of the electronic structure of the metal near the Fermi
level [Santos and Schmickler, 2007a, b, c; Santos and Schmickler, 2006].
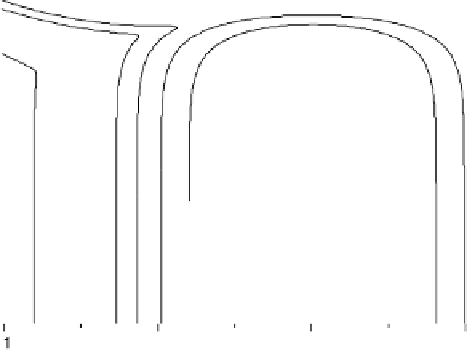
Before considering our model for electrocatalysis, it is instructive to investigate the
interaction of a single reactant orbital with a model metal containing a wide sp-band
and a narrow d-band. For this purpose, it is convenient to use the model of a semi-
elliptic band [Newns, 1969], for which several important quantities can be calculated
explicitly. A single such metal band has the form
1
=
2
2
1
1
c
w
u[w
2
(1
1
c
)
2
]
r
m
(1)
¼
1
(2
:
16)




























Search WWH ::

Custom Search