Environmental Engineering Reference
In-Depth Information
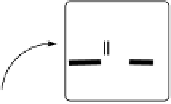
Figure 18.5 Plausible sequence of steps responsible for rapid and selective reduction of O
2
to
H
2
O by mixed-valence CcO. The square frames signify the catalytic site (Fig. 18.4c); imidazole
ligation of Cu
B
is omitted for clarity; in some or all intermediates, Cu
B
may additionally be
ligated by an exogenous ligand, such as H
2
O (in Cu
I
)orOH
2
(in Cu
II
); such ligation is not
established, and hence is omitted in all but compound P
M
and the putative hydroperoxo inter-
mediate. The dashed frames signify the noncatalytic redox cofactors. Typically used phenom-
enological names of the spectroscopically observed intermediates (compounds A, E, H, etc.) are
also indicated.
phenol of Tyr244 provides the fourth electron and a proton. Note that reduction of O
2
required no electron transfer between a ferrocytochrome c and CcO, because only
those redox forms of CcO that contain all necessary reducing equivalents at the
heme/Cu site have affinity to O
2
.
The absence of any spectroscopically observable intermediates between compound
A and compound P
M
requires electron structure computations to understand the ato-
mistic basis for rapid activation of the O - O bond in CcO. The most recently published
computational studies [Blomberg and Siegbahn, 2006], which were carried out at the
density functional theory (DFT) level (using the unrestricted B3LYP functional with
double-zbasis sets that included core potentials for Cu and Fe), suggest that this acti-
vation proceeds through a hydroperoxo intermediate, which is generated by oxidizing
Cu
B
and deprotonating the phenyl residue of Tyr244. These computations predict that
a surprisingly large barrier (13 kcal/mol) separates this hydroperoxo intermediate
from compound P
M
. Depending on the computational procedure, the hydroperoxo
intermediate has a very small to moderate (,10 kcal/mol) barrier for reversion to
compound A, making it difficult, if not impossible, to observe spectroscopically,
which is consistent with the experiment. No energy minima were found for a species

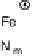
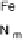



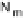
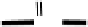

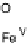

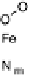



















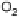
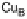
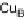






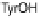
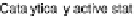









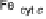
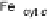








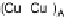
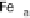




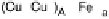



































































Search WWH ::

Custom Search