Environmental Engineering Reference
In-Depth Information
common features of all these enzymes are (i) a binuclear heme/Cu site at which O
2
is
reduced and (ii) proton pumping. All these enzymes are embedded in a membrane.
CcOs and cytochrome cbb
3
oxidases catalyze oxidation of reduced cytochrome
c (ferrocytochrome c)byO
2
. Certain CcOs can also utilize specific high-potential
iron - sulfur proteins (HiPIPs) as electron donors [Pereira et al., 1999]. Quinol oxidases
catalyze two-electron, two-proton oxidation of a stronger reductant, quinol, to quinone
by O
2
[Abramson et al., 2000].
CcOs from various organisms, as well as CcOs and quinol oxidases, are fairly
homologous structurally and functionally, and both are distinct from cytochromes
cbb
3
. Most, if not all, CcOs and quinol oxidases require two subunits for catalytic
activity (subunits I and II; Fig. 18.4), although some, such as mammalian CcO,
may contain as many as 11 more subunits of unknown functions [Abramson et al.,
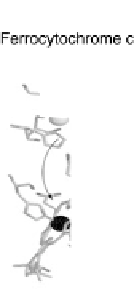
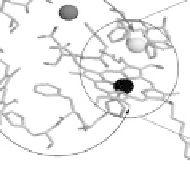
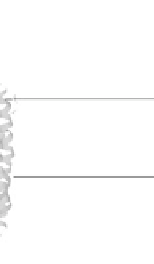
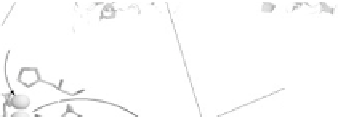
Figure 18.4 Structures of heme/Cu oxidases at different levels of detail. (a) Position of the
redox-active cofactors relative to the membrane of CcO (left, only two obligatory subunits
are shown) and quinol oxidase (right). (b) Electron transfer paths in mammalian CcO. Note
that the imidazoles that ligate six-coordinate heme a and the five-coordinate heme a
3
are
linked by a single amino acid, which can serve as a “wire” for electron transfer from ferroheme
a to ferriheme a
3
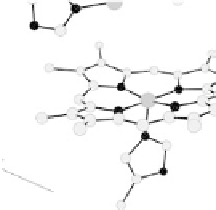
. (c) The O
2
reduction site of mammalian CcO; the numbering of the residues
corresponds to that in the crystal structure of bovine heart CcO. The subscript 3 in heme a
3
and
heme o
3
signifies the heme that binds O
2
. The structures were generated using coordinates
deposited in the Protein Data Bank, 1ar1 [Ostermeier et al., 1997]; 1fft [Abramson et al.,
2000] (a) and 1occ [Tsukihara et al., 1996] (b, c).






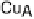

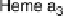











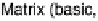
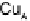


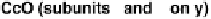








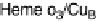





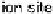



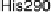
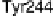



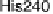
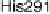





















Search WWH ::

Custom Search