Environmental Engineering Reference
In-Depth Information
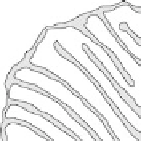
Figure 18.3 (a) Schematic representation of a mitochondrion, which has two distinct com-
partments (matrix and intermembrane space), separated by the inner mitochondrial membrane.
(b) Schematic representation of a mammalian respiratory chain, which is embedded in the inner
mitochondrial membrane. The chain comprises three sets of redox reactions, mediated by at least
three enzymes. Electron transfer from the most reduced electron carrier (NADH) to lipid-soluble
quinone is mediated by NADH dehydrogenase, and generates NAD
þ
and quinol (see Fig. 18.2
for the chemical structure of the quinol). Quinol is oxidized back to quinone by ferricytochrome
c at the enzyme cytochrome bc
1
. The resultant ferrocytochrome c is the weakest biological
reductant of the chain, and can only be oxidized by the terminal oxidant, O
2
. The reaction is
catalyzed by cytochrome c oxidase. Although thermodynamically favorable, direct reactions
between various electron donors of the chain are slow. The respiratory enzymes capture a
part of the free energy of each redox reaction by translocating protons from the matrix, which
is deficient in H
þ
, to the intermembrane space (gray block arrows). The thermodynamically
favorable backflow of H
þ
can only occur through ATP synthase, the enzyme that converts
the free energy of this flow into ATP. White and gray block arrows depict exergonic and ender-
gonic processes, respectively.
classes: the well-studied superfamily of heme/Cu oxidases [Hosler et al., 2006;
Brzezinski and Adelroth, 2006; Branden et al., 2006; Wikstrom, 2004; Pitcher and
Watmough, 2004; Pereira et al., 2001; Mills, 2000; Michel, 1999; Rich and Moody,
1997] and a less well-understood group of cytochrome bd oxidases [Junemann, 1997].
Heme/Cu oxidases are widely distributed throughout the biosphere, whereas cyto-
chromes bd are limited to certain prokaryotes (unicellular organisms lacking organells
or nucleus). Heme/Cu oxidases contain a binuclear heme/Cu site, whereas the catalytic
site in cytochromes bd is composed of two hemes. Cytochromes bd have been reviewed
in the context of fuel cells elsewhere [Boulatov, 2006], and are not discussed here.
18.2.2 ORR Catalysis by Heme
/
Cu Terminal Oxidases
Three distinct types of heme/Cu oxidases are currently recognized: cytochrome c
oxidases (CcOs or COX), quinol oxidases, and cytochrome cbb
3
oxidases [Pitcher
and Watmough, 2004], with CcOs being limited to eukaryotic organisms. The

























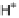

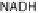
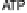
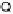

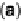











Search WWH ::

Custom Search