Environmental Engineering Reference
In-Depth Information
Figure 2.9 Model potential energy surface for combined electron and proton transfer. Q
1
is
the solvent coordinate for electron transfer and Q
2
that for proton transfer. (See color insert.)
involve a reorganization of the solvent, and the reaction coordinate can be identified with
the corresponding solvent coordinates. This idea has been explored in a recent model
[Grimminger et al., 2005] that allows the construction of model potential surfaces.
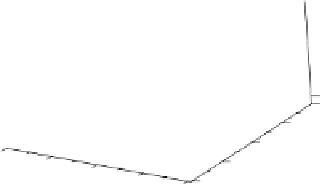
An example is shown in Fig. 2.9 (Plate 2.2); the four minima correspond to the four cor-
ners of the scheme of squares. Experimental results are usually discussed in terms of a
consecutive mechanism, in which proton and electron transfer occur as separate steps;
Fig. 2.9 corresponds to this case. However, in some cases, a concerted mechanism, in
which both particles are transferred simultaneously, may predominate; Fig. 2.10
(Plate 2.3) shows a potential energy surface for this case. A concerted process would
generally involve less reorganization of the solvent, since both the initial and final
states have the same charge. In addition, the Coulomb attraction between electron and
proton transfer would favor a simultaneous exchange. Note that only the reaction
steps involving electron transfer depend on the electrode potential, since the proton is
not transferred to the electrode. Therefore, the dominant mechanism may well change
with the potential.
Figure 2.10
Potential energy surface for combined electron and proton transfer. (See color
insert.)


















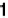

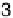

















Search WWH ::

Custom Search