Environmental Engineering Reference
In-Depth Information
17.4.2 Ethanol, Methanol, or Glycerol as Fuel
The alcohol tolerance of O
2
reduction by bilirubin oxidase means that membraneless
designs should be possible provided that the enzymes and mediators (if required) are
immoblized at the electrodes. Minteer and co-workers have made use of NAD
þ
-
dependent alcohol dehydrogenase enzymes trapped within a tetraalkylammonium
ion-exchanged Nafion film incorporating NAD
þ
/NADH for oxidation of methanol
or ethanol [Akers et al., 2005; Topcagic and Minteer, 2006]. The polymer is coated
onto an electrode modified with polymethylene green, which acts as an electrocatalyst
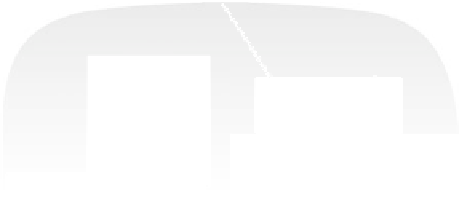
Figure 17.20 Use of enzyme catalysts in a membraneless fuel cell that operates on low levels
of H
2
in air [Vincent et al., 2006]. (a) Schematic representation of the fuel cell. PGE electrodes
modified with hydrogenase and laccase are inserted into a shallow tray of aqueous electrolyte
(pH 5 citrate solution) in contact with an atmosphere of 3% H
2
in air. Blue lines indicate pro-
ductive reactions: electrocatalytic H
2
oxidation at the anode and O
2
reduction at the cathode. Red
lines indicate unproductive reactions: consumption of electrons at the anode by direct reduction
of O
2
at bare graphite, generating radical species that may damage the enzymes, or reversible
inhibition of hydrogenase by access of O
2
to the active site. (b) Typical cell voltage/current
plot for this fuel cell obtained by applying a variable load, showing the rapid drop in current
observed at high cell voltages, consistent with high potential inactivation of the hydrogenase
at the anode. (See color insert.)






















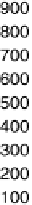
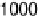

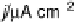
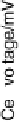

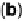
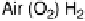
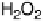
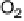











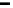



Search WWH ::

Custom Search