Environmental Engineering Reference
In-Depth Information
fuels (e.g., glucose and other sugars), O
2
, and electrolytes (primarily NaCl). In theory,
the device lifetime may be enhanced by replacing a battery with a fuel cell that
scavenges ambient fuel and oxidant.
Heller and co-workers have demonstrated a glucose/O
2
fuel cell utilizing redox
hydrogel glucose oxidase and bilirubin oxidase electrodes, operating in a grape in
which the glucose concentration exceeded 30 mM and the pH was 5.4 (Fig. 17.18)
[Mano et al., 2003]. Peak operation was obtained when the electrodes were implanted
near the skin of the grape, presumably owing to the maximized O
2
flux. The maximum
power, 240 mWcm
22
, was obtained with the cell operating at about 0.5 V, and 78% of
the initial power was retained after 24 hours of continuous operation.
Fuel cells based on unmediated electrocatalysis by heme-containing sugar
dehydrogenases have not yet been tested in biological fluids, but may be useful for
implantable applications, as they avoid the need for toxic or expensive mediators
and have minimal design constraints. Realistically, the lifetime of biofuel cells is
still
insufficient
for
biomedical
applications
requiring
surgical
installation.
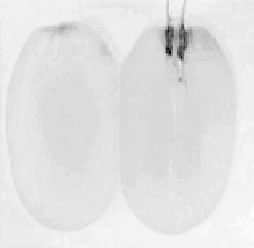
Figure 17.18 (a) Photograph of a whole and sliced grape showing the positions of implanted
carbon fiber electrodes modified with enzymes embedded in redox hydrogels (as shown in
Fig. 17.17). (b) Power output versus cell potential for the implanted fuel cell. Reprinted with
permission from Mano et al. [2003]. Copyright (2003) American Chemical Society.
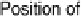

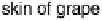

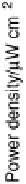
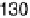
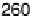




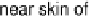
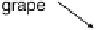











Search WWH ::

Custom Search