Environmental Engineering Reference
In-Depth Information
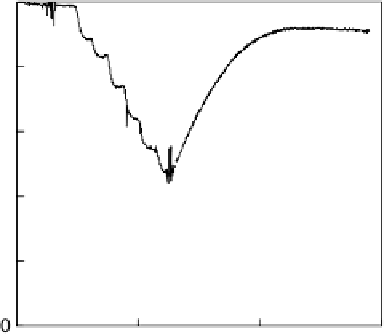
Figure 17.16 Electrocatalytic H
2
oxidation by Ralstonia metallidurans CH34 membrane-
bound hydrogenase on a PGE RDE in the presence of O
2
. The electrode is rotated at 2000
rev min
21
and polarized at
þ
0.142 V vs. SHE in buffered aqueous solution at pH 5.6 and
30 8C, close to 1 bar H
2
. Reprinted with permission from Vincent et al. [2007]. Copyright
(2007) American Chemical Society.
Thus it may prove possible to improve O
2
tolerance further by directed mutation of
residues within the protein.
Hydrogenases are typically inhibited by CO, but recover rapidly when the inhibitor
is removed. However the membrane-bound hydrogenases from Ralstonia are almost
completely insensitive to a large excess of CO [Vincent et al., 2005, 2006]. Certain
hydrogenases thus offer opportunities for oxidation of H
2
in heavily contaminated
gas streams. Sulfides have been shown to react with hydrogenases only at high poten-
tial, inhibiting H
2
oxidation above about 0 V vs. SHE, and thus would not interfere
in fuel cell catalysis under typical operating conditions.
17.4 WORKING EXAMPLES OF ENZYMES AS FUEL
CELL CATALYSTS: POSSIBILITIES FOR NOVEL DESIGNS
AND APPLICATIONS
It is important to keep in mind that exploitation of metalloenzymes in catalysis is an
emerging area, and the innovative concepts demonstrated by biofuel cells are far
more significant than the magnitude of the power outputs recorded. Demonstrations
of enzyme catalysis in fuel cells have generally been on a small scale, with power out-
puts typically in the microwatt to milliwatt range. Although these levels compare
poorly with those of conventional fuel cells, advances in the attachment of enzymes
to electrodes and the use of porous electrodes or electrodes modified with nanotubes
or carbon powders are providing improvements in current suggesting that it should be
possible to close that gap. Furthermore, niches exist where enzyme fuel cells are at an




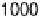
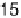


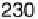
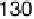
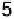
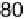
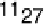
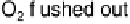
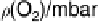











Search WWH ::

Custom Search