Environmental Engineering Reference
In-Depth Information
2.4 PROTON TRANSFER
Just like the electron, the proton is an elementary particle, which in an aqueous sol-
ution never exists just by itself. It associates with water, forming species such as the
Eigen H
3
O
þ
[Eigen, 1963] or the Zundel H
5
O
2
[Zundel and Metzger, 1968] ion,
and exchanges rapidly with the hydrogen atoms of the water molecules. Its hydration
energy is extremely high, of the order of 12 eV, and so is the ionization energy of the
hydrogen atom (about 13.6 eV). Thus, the ionization of a hydrogen atom to form a
proton requires much energy, but the gain in solvation energy is of the same order
of magnitude. Because of its quantum nature, and its rapid exchange with water, the
transport of a proton towards an electrode surface cannot be viewed as the motion
of an individual particle, but must occur by a Grotthus-type mechanism. It thus differs
essentially from the transfer of a simple ion, which we have discussed above. In par-
ticular, it cannot involve a barrier amounting to half of its solvation energy, since this
would be far too high to allow the proton to reach the surface.
Besides these generalities, little is known about proton transfer towards an electrode
surface. Based on classical molecular dynamics, it has been suggested that the rate-
determining step is the orientation of the H
3
O
þ
with one proton towards the surface
[Pecina and Schmickler, 1998]; this would be in line with proton transport in bulk
water, where the proton transfer itself occurs without a barrier, once the participating
molecules have a suitable orientation. This is also supported by a recent quantum
chemical study of hydrogen evolution on a Pt(111) surface [Skulason et al., 2007],
in which the barrier for proton transfer to the surface was found to be lower than
0.15 eV. This extensive study used a highly idealized model for the solution—a bilayer
of water with a few protons added—and it is not clear how this simplification affects the
result. However, a fully quantum chemical model must necessarily limit the number of
particles, and this study is probably among the best that one can do at present.
Of course, proton transfer can also occur between two reactants in the solution. As
such, it is not an electrochemical reaction, unless it is combined with an electron
exchange with the electrode. Such a combined electron - proton transfer can be rep-
resented by the scheme of squares shown in Fig. 2.8. Both electron and proton transfer
Figure 2.8
Reaction scheme for combined electron and proton transfer.



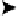



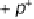
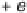














Search WWH ::

Custom Search