Environmental Engineering Reference
In-Depth Information
the film is prepared on a porous conducting support such as carbon nanotubes or
carbon paper [Barton et al., 2007].
A rational strategy for direct attachment of laccase to graphite surfaces was reported
recently [Blanford et al., 2007]. As described above, the crystallographic structure of
T. versicolor laccase III reveals a wide, hydrophobic pocket at the surface of the
protein close to the mononuclear type I Cu center at which organic substrates bind
[Bertrand et al., 2002]. The proximity of the pocket to the “blue” Cu center provides
a route for electrons to be transferred through the protein to the trinuclear active site
where O
2
is reduced to water. A pyrolytic graphite electrode modified with 2-amino-
anthracene (by electrochemical reduction of the anthracene-2-diazonium ion) forms
a surface for direct adsorption of laccase. The laccase-modified electrode shows a
well-defined electrocatalytic wave in the presence of O
2
, with O
2
reduction commen-
cing above 850 mV with respect to a standard hydrogen electrode (SHE) at pH 4
(Fig. 17.7) (Plate 17.3). The magnitude and long-term stability of the current response
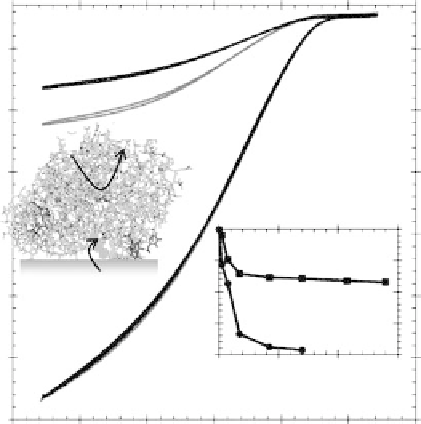
Figure 17.7 Electrocatalysis of O
2
reduction by Pycnoporus cinnabarinus laccase on a
2-aminoanthracene-modified pyrolytic graphite edge (PGE) electrode and an unmodified
PGE electrode at 25 8C in sodium citrate buffer (200 mM, pH 4). Red curves were recorded
immediately after spotting laccase solution onto the electrode, while black curves were recorded
after exchanging the electrochemical cell solution for enzyme-free buffer solution. Insets show
the long-term percentage change in limiting current (at 0.44 V vs. SHE) for electrocatalytic O
2
reduction by laccase on an unmodified PGE electrode (†) or a 2-aminoanthracene modified elec-
trode (
B
) after storage at 4 8C, and a cartoon representation of the probable route for electron trans-
fer through the anthracene (shown in blue) to the “blue” Cu center of laccase. Reproduced by
permission of The Royal Society of Chemistry from Blanford et al., 2007. (See color insert.)















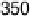


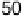


































Search WWH ::

Custom Search